추천 제품
Quality Level
제품 라인
ReagentPlus®
분석
99%
bp
170-172 °C/10 mmHg (lit.)
mp
105-107 °C (lit.)
solubility
95% ethanol: soluble 50 mg/mL, clear, colorless to faintly yellow
작용기
carboxylic acid
SMILES string
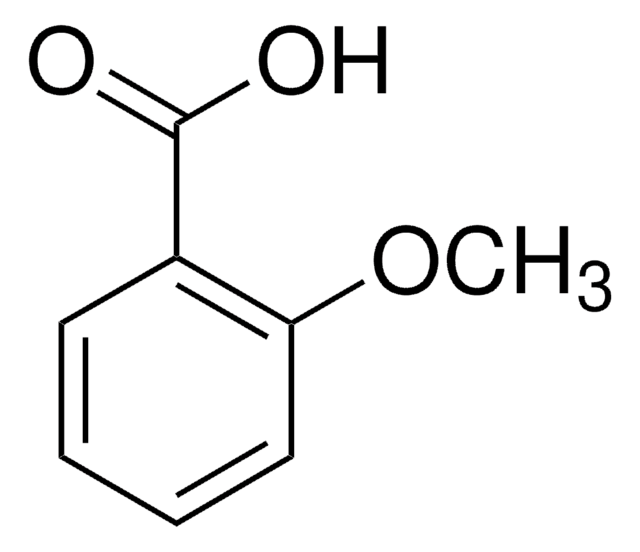
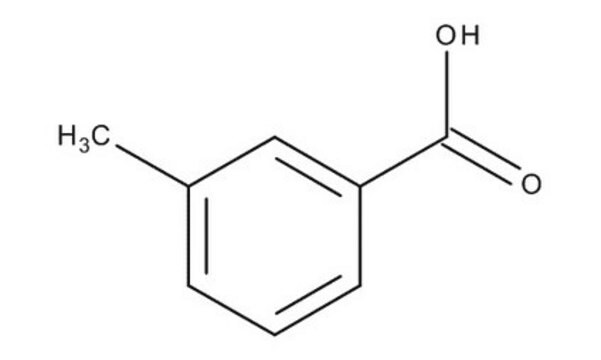
COc1cccc(c1)C(O)=O
InChI
1S/C8H8O3/c1-11-7-4-2-3-6(5-7)8(9)10/h2-5H,1H3,(H,9,10)
InChI key
XHQZJYCNDZAGLW-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
3-Methoxybenzoic acid is an important intermediate in the synthesis of natural products.
애플리케이션
3-Methoxybenzoic acid was used in the synthesis and characterization of 3-methoxybenzoates of europium (III) and gadolinium (III). It was used in conversion of aromatic carboxylic acids into methyl esters and reduction to the corresponding primary alcohols using a sodium borohydride-THF-methanol system.
법적 정보
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Synthesis, characterization and thermal behaviour of solid-state compounds of europium (III) and gadolinium (III) 3-methoxybenzoate.
Dametto PR, et al.
Journal of Thermal Analysis and Calorimetry, 97(2), 765-768 (2009)
Sodium borohydride reduction of aromatic carboxylic acids via methyl esters.
Saeed A and Ashraf Z.
Journal of Chemical Sciences (Bangalore), 118(5), 419-423 (2006)
K A DeWeerd et al.
Applied and environmental microbiology, 54(5), 1237-1242 (1988-05-01)
O-methyl substituents of aromatic compounds can provide C1 growth substrates for facultative and strict anaerobic bacteria isolated from diverse environments. The mechanism of the bioconversion of methoxylated benzoic acids to the hydroxylated derivatives was investigated with a model substrate and
N Dodoff et al.
Journal of inorganic biochemistry, 54(3), 221-233 (1994-05-15)
The complexes [Pt(bah)2X2], [Pt(NH3)(bah)Cl2].0.5H2O, [Pt(mbah)2X2], and [Pt(NH3)(mbah)Cl2] (bah = benzoic acid hydrazide, mbah = 3-methoxybenzoic acid hydrazide; X = Cl, Br, I) have been prepared and characterized by elemental analysis, electric conductivity, IR, 1H NMR, and electronic spectra. A cis-square
Thi-Huu Nguyen et al.
Organic letters, 7(12), 2445-2448 (2005-06-04)
[reaction: see text] If employed in THF at 0 degrees C, LTMP metalates meta-anisic acid at the doubly activated position. In contrast, n-BuLi/t-BuOK deprotonates position C-4 preferentially at low temperature. Functionalization at C-6 requires protection of the C-2 site beforehand.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.





![Benzo[a]fluorenone BCR®, certified reference material](/deepweb/assets/sigmaaldrich/product/structures/881/090/eae85258-97ed-4de7-90c1-c0e0e495552e/640/eae85258-97ed-4de7-90c1-c0e0e495552e.png)