124230
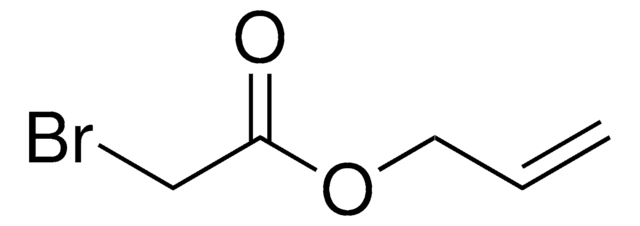
tert-Butyl bromoacetate
98%
동의어(들):
Bromoacetic acid tert-butyl ester, t-Butyl bromoacetate, tert-Butyl 2-bromoacetate
로그인조직 및 계약 가격 보기
모든 사진(3)
About This Item
Linear Formula:
BrCH2COOC(CH3)3
CAS Number:
Molecular Weight:
195.05
Beilstein:
1753010
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
98%
양식
liquid
refractive index
n20/D 1.445 (lit.)
bp
50 °C/10 mmHg (lit.)
density
1.321 g/mL at 25 °C (lit.)
작용기
bromo
ester
SMILES string
CC(C)(C)OC(=O)CBr
InChI
1S/C6H11BrO2/c1-6(2,3)9-5(8)4-7/h4H2,1-3H3
InChI key
BNWCETAHAJSBFG-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
tert-Butyl bromoacetate serves as building blocks during the synthesis of model N-substituted oligoglycines (peptoids) containing an N-linked lactoside side-chain.
애플리케이션
tert-Butyl bromoacetate has been used in the synthesis of:
- nitrilotriacetic acid end-functionalized polystyrene by atom transfer radical polymerization
- building block for substituted t-butyl acetates
- dihydropyranyl prelinker which is useful in polymer-assisted deprotection of oligosacchararides
- collagenase inhibitor (S,S,R)-(-)-actinonin It is the starting reagent for the synthesis of N-Oxalylglycine derivatives.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
120.2 °F - closed cup
Flash Point (°C)
49 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
Efficient polymer-assisted strategy for the deprotection of protected oligosaccharides.
Hiroshi Tanaka et al.
Angewandte Chemie (International ed. in English), 45(38), 6349-6352 (2006-08-19)
Synthesis of new glycopeptidomimetics based on N-substituted oligoglycine bearing an N-linked lactoside side-chain.
Saha UK and Roy R.
Journal of the Chemical Society. Chemical Communications, 24, 2571-2573 (1995)
SYNTHESIS OF (NITRILOTRIACETIC ACID)-END-FUNCTIONALIZED POLYSTYRENE USING ATOM TRANSFER RADICAL POLYMERIZATION.
Cho HY, et al.
Synthesis, 1000, 4H-4H (2006)
Journal of the Chemical Society. Perkin Transactions 1, 459-459 (1993)
Guillaume Jeannotte et al.
The Journal of organic chemistry, 69(14), 4656-4662 (2004-07-03)
Fused heteroarylprolines were prepared starting from 4-oxo-N-(PhF)proline benzyl ester (6, PhF = 9-(9-phenylfluorenyl)) following two approaches. First, allylation of oxoproline 6 followed by Wacker oxidation gave 1,4-dione 8 that was selectively converted to pyrroloproline 10b, pyrrolopyrrole 12, and pyridazinoproline 9.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.




![Bicyclo[2.2.1]hept-2-ene 99%](/deepweb/assets/sigmaaldrich/product/structures/270/492/95fd4909-6108-4858-8c94-609b54387149/640/95fd4909-6108-4858-8c94-609b54387149.png)