138622
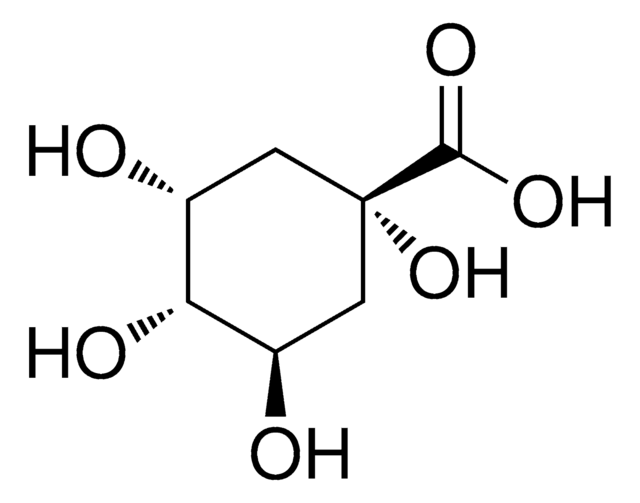
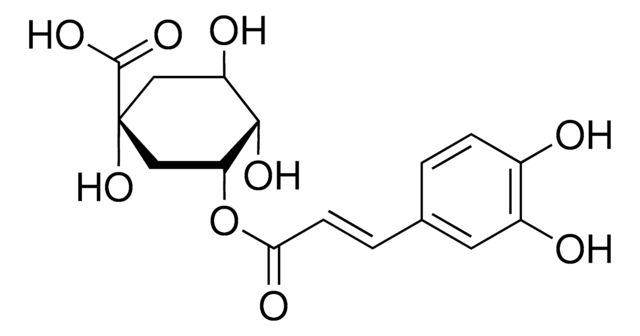
D-(−)-Quinic acid
98%
동의어(들):
(-)-Quinic acid, (1alpha,3R,4alpha,5R)-1,3,4,5-Tetrahydroxycyclohexanecarboxylic acid, D-(-)-Quinic acid
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C7H12O6
CAS Number:
Molecular Weight:
192.17
Beilstein:
2212412
EC Number:
MDL number:
UNSPSC 코드:
51113400
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
98%
형태
powder
광학 활성
[α]20/D −43.9°, c = 11.2 in H2O
작용기
carboxylic acid
hydroxyl
SMILES string
O[C@@H]1C[C@@](O)(C[C@@H](O)[C@H]1O)C(O)=O
InChI
1S/C7H12O6/c8-3-1-7(13,6(11)12)2-4(9)5(3)10/h3-5,8-10,13H,1-2H2,(H,11,12)/t3-,4-,5-,7+/m1/s1
InChI key
AAWZDTNXLSGCEK-WYWMIBKRSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
D-(-)-Quinic acid, a plant metabolite, is chiral building block used in multistep chemical synthesis of natural compounds.
애플리케이션
D-(−)-Quinic acid can be used as:
- A chiral selector electrolyte along with copper(II) sulfate. This electrolyte is utilized in chiral resolution DL-tartaric acid by ligand-exchange capillary electrophoresis method.
- A starting material in the synthesis of stereoisomers of 3,4,6-trihydroxyazepanes, 7-hydroxymethyl-3,4,5-trihydroxyazepanes, and 3,4,5-trihydroxyazepanes, as potential inhibitors of glycosidase.
- A precursor for the preparation of trihydroxy piperidine derivatives and (+)-proto-quercitol glycosidase inhibitors.
D-(-)-Quinic acid has been used as a standard to determine the composition of organic acids in bitter gentian teas and in developing cranberry fruit by HPLC. It may be used in the preparation of 3,4-O-isopropylidene-3(R),4(S)-dihydroxycyclohexanone.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Tzenge-Lien Shih et al.
The Journal of organic chemistry, 72(11), 4258-4261 (2007-05-08)
Several new stereoisomers of 3,4,6-trihydroxyazepanes and 7-hydroxymethyl-3,4,5-trihydroxyazepanes as well as known 3,4,5-trihydroxyazepanes were synthesized as potent glycosidase inhibitors from D-(-)-quinic acid in an efficient manner. The key step employs dihydroxylation of protected chiral 1,4,5-cyclohex-2-enetriols under RuCl3/NaIO4/phosphate buffer (pH 7) condition
d-(-)-Quinic acid: a chiron store for natural product synthesis
Barco A, et al
Tetrahedron Asymmetry, 8(21), 3515-3545 (1997)
A unified asymmetric approach to substituted hexahydroazepine and 7-azabicyclo [2.2. 1] heptane ring systems from D (-)-quinic acid: Application to the formal synthesis of (-)-balanol and (-)-epibatidine
Albertini E, et al
Tetrahedron Letters, 38(4), 681-684 (1997)
Qiuling Li et al.
G3 (Bethesda, Md.), 6(10), 3351-3359 (2016-08-26)
Drosophila melanogaster is a powerful model organism for dissecting the molecular mechanisms that regulate sleep, and numerous studies in the fly have identified genes that impact sleep-wake cycles. Conditional genetic analysis is essential to distinguish the mechanisms by which these
A facile synthesis of a new trihydroxy piperidine derivative and (+)-proto-quercitol from d-(-)-quinic acid
Shih T-L, et al.
Tetrahedron Letters, 45(29), 5751-5754 (2004)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.