모든 사진(2)
About This Item
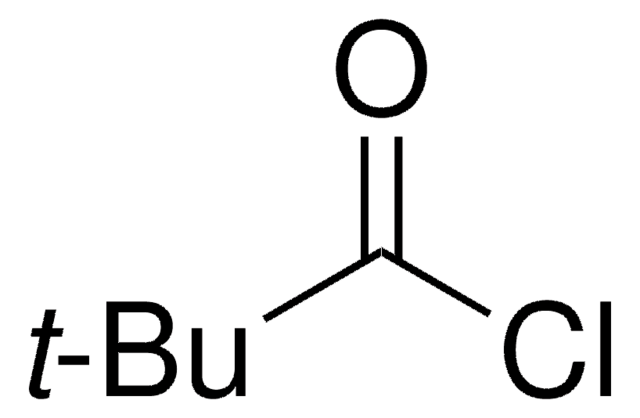
Linear Formula:
(CH3)3CCOOCH2Cl
CAS Number:
Molecular Weight:
150.60
Beilstein:
1560838
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
97%
양식
liquid
refractive index
n20/D 1.417 (lit.)
bp
146-148 °C (lit.)
density
1.045 g/mL at 25 °C (lit.)
작용기
chloro
ester
SMILES string
CC(C)(C)C(=O)OCCl
InChI
1S/C6H11ClO2/c1-6(2,3)5(8)9-4-7/h4H2,1-3H3
InChI key
GGRHYQCXXYLUTL-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Chloromethyl pivalate reacts with sodium salt of sulbactam to yield sulbactam pivoxil. It undergoes acylation reaction with 9-(2-phosphonylmethoxyethyl)adenine (PMEA) to yield bis(pivaloyloxymethyl) PMEA.
애플리케이션
Chloromethyl pivalate was used in the synthesis of pivaloyloxy methyl ester of ofloxacin as prodrug. It was used as the reagent during the synthesis of an isoindoline-annulated, tricyclic sultam library via microwave-assisted, continuous-flow organic synthesis.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
104.0 °F - closed cup
Flash Point (°C)
40 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
L S Changov et al.
Farmaco (Societa chimica italiana : 1989), 55(2), 134-135 (2000-04-27)
Sulbactam pivoxil, a prodrug of the beta-lactamase inhibitor sulbactam, was prepared in high yield by reacting the sodium salt of sulbactam with chloromethyl pivalate in a polar solvent, then diluting the reaction mixture with water and isolating the product by
J E Starrett et al.
Antiviral research, 19(3), 267-273 (1992-09-11)
9-(2-Phosphonylmethoxyethyl)adenine (PMEA; 1) was acylated with chloromethyl pivalate to afford bis(pivaloyloxymethyl) PMEA (2). The ester prodrug demonstrated enhanced in vitro potency against HSV-2 greater than 150-fold higher than the parent compound. The antiviral activity of 2 was 50-fold better than
Y Maeda et al.
Biological & pharmaceutical bulletin, 16(6), 594-599 (1993-06-01)
We newly synthesized a pivaloyloxymethyl ester of ofloxacin (OFLX-PVM) as prodrug in order to avoid the chelate formation between new quinolone and metal cations such as Al3+, Mg2+, Ca2+, or Fe2+ in the gastrointestinal tract. This compound was rapidly hydrolyzed
Farman Ullah et al.
Synthesis, 44(16), doi:10-doi:10 (2012-01-01)
A microwave-assisted, continuous-flow organic synthesis (MACOS) protocol for the synthesis of an isoindoline-annulat-ed, tricyclic sultam library, utilizing a Heck-aza-Michael (HaM) strategy, is reported. This sequence involves a Heck reaction on vi-nylsulfonamides with batch microwave heating followed by a one-pot, sequential
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.