모든 사진(1)
About This Item
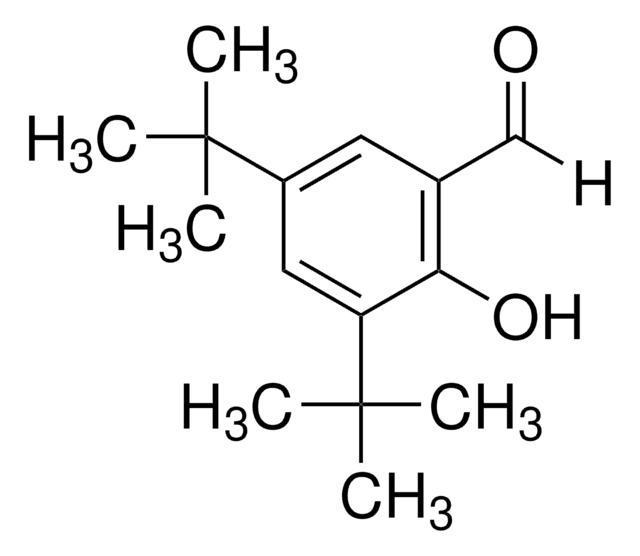
Linear Formula:
[(CH3)3C]2C6H2-2-(OH)CO2H
CAS Number:
Molecular Weight:
250.33
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
97%
mp
157-162 °C (lit.)
작용기
carboxylic acid
SMILES string
CC(C)(C)c1cc(C(O)=O)c(O)c(c1)C(C)(C)C
InChI
1S/C15H22O3/c1-14(2,3)9-7-10(13(17)18)12(16)11(8-9)15(4,5)6/h7-8,16H,1-6H3,(H,17,18)
InChI key
ZWQBZEFLFSFEOS-UHFFFAOYSA-N
애플리케이션
3,5-Di-tert-butylsalicylic acid was used to study long wavelength fluorescence emission of 3,5-Di-tert-butylsalicylic acid in a variety of organic solvents. It was also used to catalyze the reaction between aldehydes and silyl ketene acetals.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
354.2 °F - closed cup
Flash Point (°C)
179 °C - closed cup
개인 보호 장비
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
A first example of macromolecular Ti (IV) Lewis acid in the catalytic enantioselective Mukaiyama reaction.
Tetrahedron Asymmetry, 9(9), 1479-1482 (1998)
Veli T Kasumov et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 107, 31-38 (2013-02-19)
A series of new polyfluorinated palladium(II) complexes (7-12) of N-polyfluorophenyl-3,5-di-tert-butylsalicylaldimines (1-6) have been synthesized. They were characterized by analytical, spectroscopic (UV/Vis, IR, (1)H NMR, and ESR), electrochemical methods and their chemical oxidation and hydrogenation catalytic activity were studied. The X-ray
Photoinduced proton transfers in 3, 5-di-tert-butylsalicylic acid.
The Journal of Physical Chemistry, 99(32), 12103-12108 (1995)
M V Chidambaram et al.
Journal of pharmaceutical sciences, 80(8), 810-811 (1991-08-01)
The initial yield of 3,5-di-t-butylsalicylic acid obtained via Kolbe-Schmitt carboxylation of the potassium salt of 2,4-di-t-butylphenol was less than 1% and was accompanied by a 65% yield of 2,2'-dihydroxy-3,3',5,5'- tetra-t-butylbiphenyl, a dimer of the 2,4-di-t-butylphenol formed by ortho coupling of
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.