15404
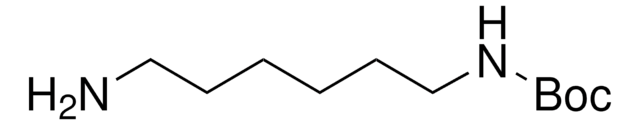
N-Boc-1,4-butanediamine
≥97.0% (GC/NT)
동의어(들):
N-Boc-1,4-diaminobutane, tert-Butyl N-(4-aminobutyl)carbamate
About This Item
추천 제품
Quality Level
분석
≥97.0% (GC/NT)
반응 적합성
reagent type: cross-linking reagent
refractive index
n20/D 1.460
density
0.984 g/mL at 20 °C (lit.)
작용기
Boc
amine
SMILES string
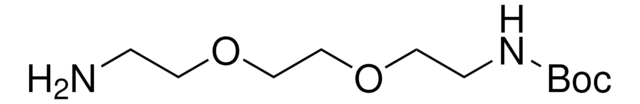
NCCCCNC(OC(C)(C)C)=O
InChI
1S/C9H20N2O2/c1-9(2,3)13-8(12)11-7-5-4-6-10/h4-7,10H2,1-3H3,(H,11,12)
InChI key
ZFQWJXFJJZUVPI-UHFFFAOYSA-N
애플리케이션
- Carboxy-Silane Coated Iron Oxide Nanoparticles: Details the application of N-Boc-1,4-butanediamine in modifying iron oxide nanoparticles for imaging and drug delivery (D Stanicki, S Boutry, S Laurent, et al., 2014). Access the article.
기타 정보
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
228.2 °F - closed cup
Flash Point (°C)
109.0 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
문서
Mono-Boc-protected diamines are versatile building blocks for chemical synthesis. Their production is a lot more challenging than the simple reaction scheme might imply, because the Boc-anhydride reagent cannot differentiate between the two identical amino moieties in the substrate.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.