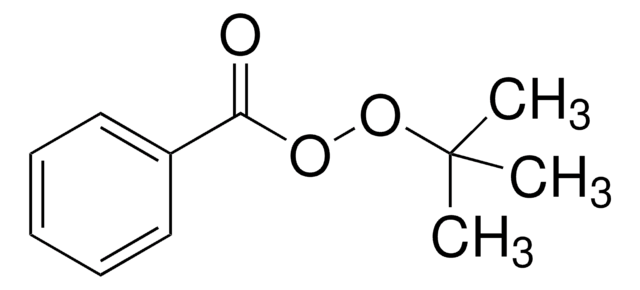
추천 제품
vapor density
6.7 (vs air)
Quality Level
vapor pressure
3.36 mmHg ( 50 °C)
분석
98%
양식
liquid
refractive index
n20/D 1.499 (lit.)
bp
75-76 °C/0.2 mmHg (lit.)
solubility
water: soluble 1.18 g/L
density
1.021 g/mL at 25 °C (lit.)
작용기
peroxide
phenyl
SMILES string
CC(C)(C)OOC(=O)c1ccccc1
InChI
1S/C11H14O3/c1-11(2,3)14-13-10(12)9-7-5-4-6-8-9/h4-8H,1-3H3
InChI key
GJBRNHKUVLOCEB-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
tert-butyl peroxybenzoate (TBPB)-mediated 2-isocyanobiaryl insertion with 1,4-dioxane has been reported.
애플리케이션
Luperox was employed as polymerization and cross-linking catalyst. It was also was employed as initiator during:
- grafting of 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO)-4-oxyacetamido-(3 propyltriethoxysilane) to poly(ethylene co-octene)
- preparation of conformal poly(cyclohexyl methacrylate) thin films via initiated chemical vapor deposition
법적 정보
Product of Arkema Inc.
Luperox is a registered trademark of Arkema Inc.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Inhalation - Aquatic Acute 1 - Aquatic Chronic 3 - Org. Perox. C - Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
4.1A - Other explosive hazardous materials
WGK
WGK 2
Flash Point (°F)
200.1 °F - closed cup
Flash Point (°C)
93.4 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Structural comparison of products from peroxide-initiated grafting of vinylsilane and silane-functionalized nitroxyl to hydrocarbon and polyolefin substrates.
Weaver JD, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 46(13), 4542-4555 (2008)
Chengguo Liu et al.
Polymers, 11(5) (2019-05-10)
New tung oil (TO)-based, unsaturated, co-ester (Co-UE) macromonomers bearing steric hindrance were synthesized by modifying a TO-based maleate (TOPERMA) monomer with an anhydride structure with hydroxyethyl methacrylate (HEMA) and methallyl alcohol (MAA), respectively. The obtained Co-UE monomers (TOPERMA-HEMA and TOPERMA-MAA)
Jia-Jia Cao et al.
Chemical communications (Cambridge, England), 50(49), 6439-6442 (2014-04-05)
An efficient method for the construction of 6-alkyl phenanthridines by tert-butyl peroxybenzoate (TBPB)-mediated 2-isocyanobiaryl insertion with 1,4-dioxane was established. Two new C-C bonds were formed in this reaction via a sequential C(sp(3))-H/C(sp(2))-H bond functionalization under metal-free conditions.
Jingjing Xu et al.
ACS applied materials & interfaces, 3(7), 2410-2416 (2011-06-08)
Conformal poly(cyclohexyl methacrylate) (pCHMA) thin films were synthesized via initiated chemical vapor deposition (iCVD), with tert-butyl peroxybenzoate (TBPOB) as the initiator, representing the first time that TBPOB has been used as an initiator for iCVD synthesis. Using TBPOB instead of
M Athar et al.
Carcinogenesis, 10(8), 1499-1503 (1989-08-01)
Humans are exposed to various peroxy and hydroperoxy compounds which are in use in the cosmetic, pharmaceutical and polymer industries and which are also generated as a result of the peroxidative metabolic conversion of certain lipids. This study was designed
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.