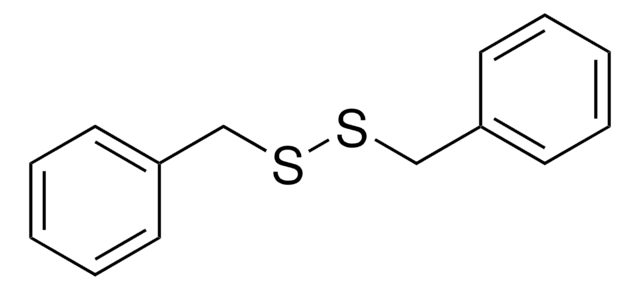
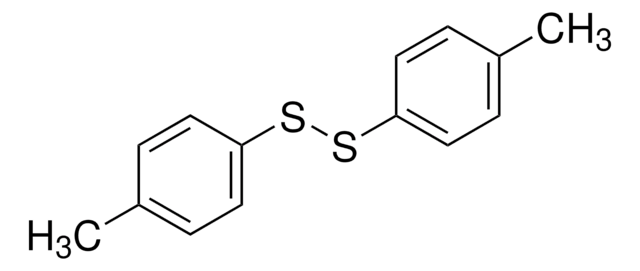
추천 제품
Quality Level
분석
99%
양식
powder
mp
58-60 °C (lit.)
solubility
xylene: soluble 3%, clear, colorless to yellow
작용기
disulfide
SMILES string
S(Sc1ccccc1)c2ccccc2
InChI
1S/C12H10S2/c1-3-7-11(8-4-1)13-14-12-9-5-2-6-10-12/h1-10H
InChI key
GUUVPOWQJOLRAS-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Phenyl disulfide is used as a precursor for the synthesis of phenyl selenosulfide (PhS-SePh), which is vital in Li-ion battery production.
애플리케이션
Phenyl disulfide is the hydrolysis product of dyfonate( insecticide). Phenyl disulfide (diphenyl disulphide) participates in hydrothiolation of alkynes via amine-mediated single electron transfer mechanism.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Identification of hydrolytic metabolites of dyfonate in alkaline aqueous solutions by using high performance liquid chromatography-UV detection and gas chromatography-mass spectrometry.
Wang T, et al.
International Journal of Environmental Analytical Chemistry, 90(12), 948-961 (2010)
Mixture is better: enhanced electrochemical performance of phenyl selenosulfide in rechargeable lithium batteries
Guo, Wei and Bhargav
Journal of the Chemical Society. Chemical Communications, 54, 8873-8876 (2018)
Molecular modeling and enzyme kinetics indicate a novel mechanism for mammalian 5-lipoxygenase.
R W Egan et al.
Advances in prostaglandin, thromboxane, and leukotriene research, 17A, 69-74 (1987-01-01)
J I Rossato et al.
Neurochemical research, 27(4), 297-303 (2002-04-18)
Ebselen (2-phenyl- 1,2-benzisoselenazole-3 (2H)-one) is a seleno-organic compound with antioxidant properties, and anti-inflammatory actions. Recently, ebselen improved the outcome of acute ischemic stroke in humans. In the present study, the potential antioxidant capacity of organochalcogenide compounds diphenyl diselenide (PhSe)2, diphenyl
C W Nogueira et al.
Toxicology, 191(2-3), 169-178 (2003-09-11)
Organochalcogens are important intermediates and useful reagents in organic synthesis, which can increase human exposure risk to these chemicals in the workplace. As well, there are a number of reported cases of acute toxicity following organochalcogen ingestion of vitamins and
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.