추천 제품
Quality Level
분석
99%
양식
liquid
refractive index
n20/D 1.521 (lit.)
bp
113 °C/9 mmHg (lit.)
mp
−2-−1 °C (lit.)
density
1.001 g/mL at 25 °C (lit.)
작용기
nitrile
phenyl
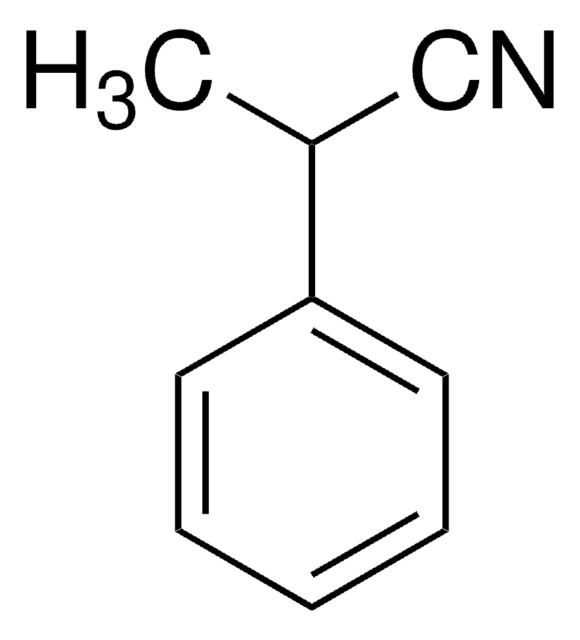
SMILES string
N#CCCc1ccccc1
InChI
1S/C9H9N/c10-8-4-7-9-5-2-1-3-6-9/h1-3,5-6H,4,7H2
InChI key
ACRWYXSKEHUQDB-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
The effect of 3-Phenylpropionitrile on the growth and survival of woodlouse Porcellio scaber has been studied.
애플리케이션
3-Phenylpropionitrile was used to study the free enzyme activity of nitrilase AtNIT1.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
230.0 °F - closed cup
Flash Point (°C)
110 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Selective hydrolysis of the nitrile group of cis-dihydrodiols from aromatic nitriles.
Journal of Molecular Catalysis. B, Enzymatic, 38(2), 78-83 (2006)
Marijana Popović et al.
Biomolecules, 10(2) (2020-02-27)
Horseradish degradation products, mainly isothiocyanates (ITC) and nitriles, along with their precursors glucosinolates, were characterized by GC-MS and UHPLC-MS/MS, respectively. Volatiles from horseradish leaves and roots were isolated using microwave assisted-distillation (MAD), microwave hydrodiffusion and gravity (MHG) and hydrodistillation (HD).
Yu-Chun Chiu et al.
International journal of molecular sciences, 19(4) (2018-04-05)
Methyl jasmonate (MeJA), synthesized in the jasmonic acid (JA) pathway, has been found to upregulate glucosinolate (GS) biosynthesis in plant species of the Brassicaceae family. Exogenous application of MeJA has shown to increase tissue GS concentrations and the formation of
Yu-Chun Chiu et al.
Foods (Basel, Switzerland), 9(6) (2020-06-12)
Exogenous methyl jasmonate (MeJA) treatment was known to increase the levels of neoglucobrassicin and their bioactive hydrolysis products in broccoli (Brassica oleracea var. italica), but the fate of MeJA-induced glucosinolates (GSLs) after various cooking methods was unknown. This study measured
A E Elaine van Ommen Kloeke et al.
Chemosphere, 89(9), 1084-1090 (2012-06-16)
Glucosinolates are compounds produced by commercial crops which can hydrolyse in a range of natural toxins that may exert detrimental effects on beneficial soil organisms. This study examined the effects of 2-phenylethyl isothiocyanate and 3-phenylpropionitrile on the survival and growth
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.