추천 제품
제품명
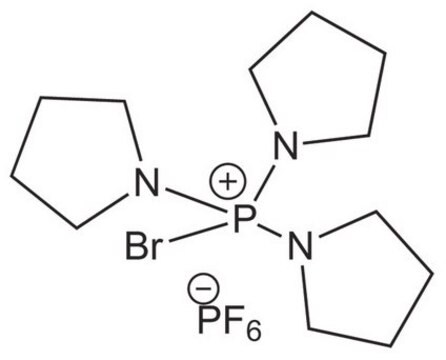
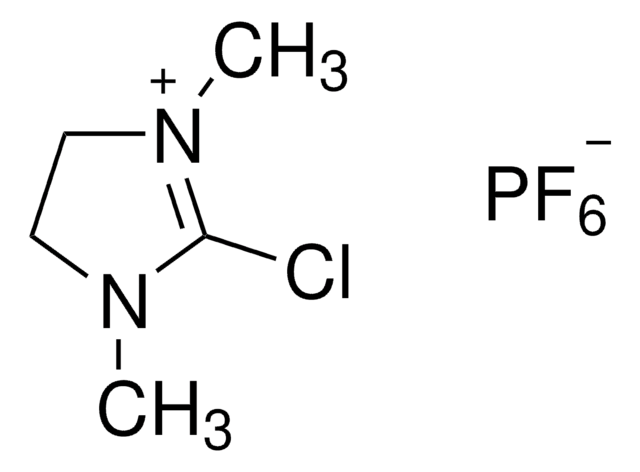
Bromotripyrrolidinophosphonium hexafluorophosphate, ≥95.0% (HPLC)
Quality Level
분석
≥95.0% (HPLC)
양식
solid
반응 적합성
reaction type: Coupling Reactions
응용 분야
peptide synthesis
저장 온도
−20°C
SMILES string
F[P-](F)(F)(F)(F)F.Br[P+](N1CCCC1)(N2CCCC2)N3CCCC3
InChI
1S/C12H24BrN3P.F6P/c13-17(14-7-1-2-8-14,15-9-3-4-10-15)16-11-5-6-12-16;1-7(2,3,4,5)6/h1-12H2;/q+1;-1
InChI key
CYKRMWNZYOIJCH-UHFFFAOYSA-N
애플리케이션
Catalyst for:
Synthesis of primary amides
Direct dehydrative phosphonium cross coupling
Direct arylation
Pyrrolidide formation by phosphonium salt coupling reagents
Synthesis of primary amides
Direct dehydrative phosphonium cross coupling
Direct arylation
Pyrrolidide formation by phosphonium salt coupling reagents
It can be used as a coupling reagent:
It can also be used as an activating reagent:
- For Suzuki–Miyaura cross-coupling of phenols and arylboronic acids to synthesize biaryls and heterobiaryls.
- To functionalize pyrimidines (synthesized from 3,4-dihydropyrimidin-2(1H)-ones (DHPMs) via the Kappe dehydrogenation) by using various nucleophiles.
- To synthesize formamidines by coupling with various primary amines and N,N-diisopropylethylamine.
It can also be used as an activating reagent:
- For the activation of C-OH bond in tautomerizable heterocycles to form the phosphonium salt which aids the Sonogashira coupling of heterocycles with various alkynes.
- For the one–pot activation of C-O bond in phenols and further coupling reaction with phosphine oxide or phosphite to form C-P bonds.
기타 정보
Reactive peptide coupling reagent suitable for α,α-dialkyl amino acids; Synthesis of depsipeptides
법적 정보
PyBroP is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Efficient Pd-Catalyzed Coupling of Tautomerizable Heterocycles with Terminal Alkynes via C-OH Bond Activation Using PyBrOP.
Shi C and Aldrich CC
Organic Letters, 12(10), 2286-2289 (2010)
T. Kurome et al.
Tetrahedron, 52, 4327-4327 (1996)
PyBroP: a convenient activator for the synthesis of formamidines.
Delarue S and Sergheraert C
Tetrahedron Letters, 40(30), 5487-5490 (1999)
Ni-catalyzed construction of C-P bonds from electron-deficient phenols via the in situ aryl C-O activation by PyBroP.
Zhao YL, et al.
Chemical Communications (Cambridge, England), 48(47), 5868-5870 (2012)
Efficient Conversion of Biginelli 3, 4-Dihydropyrimidin-2 (1 H)-one to Pyrimidines via PyBroP-Mediated Coupling.
Kang FA, et al.
The Journal of Organic Chemistry, 70(5), 1957-1960 (2005)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.