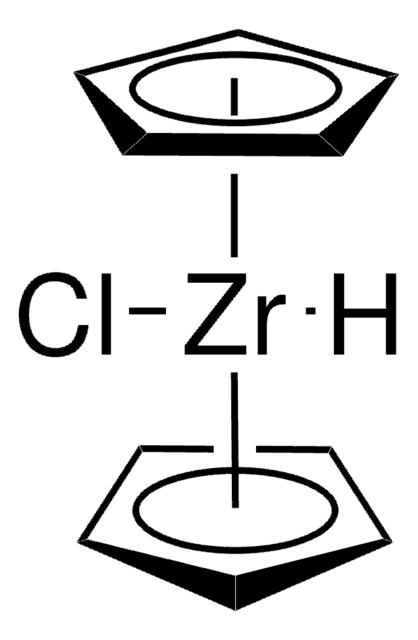
196215
Bis(cyclopentadienyl)zirconium(IV) dichloride
≥98%
동의어(들):
Dichlorodicyclopentadienylzirconium, Dichlorozirconocene, Zirconium dicyclopentadiene dichloride, Di(cyclopentadienyl)zirconium(IV) dichloride, Zirconocene dichloride
About This Item
추천 제품
Quality Level
분석
≥98%
양식
solid
반응 적합성
core: zirconium
reaction type: Polymerization Reactions
reagent type: catalyst
reaction type: Olefin Metathesis
환경친화적 대안 제품 특성
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
파라미터
moisture sensitive
mp
242-245 °C (lit.)
환경친화적 대안 카테고리
, Aligned
저장 온도
2-8°C
SMILES string
Cl[Zr]Cl.[CH]1[CH][CH][CH][CH]1.[CH]2[CH][CH][CH][CH]2
InChI
1S/2C5H5.2ClH.Zr/c2*1-2-4-5-3-1;;;/h2*1-5H;2*1H;/q;;;;+2/p-2
InChI key
QRUYYSPCOGSZGQ-UHFFFAOYSA-L
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Zirconocene dichloride serves as a catalyst, enabling efficient synthesis of pyrrole with high yields and simplifies the purification process economically.
애플리케이션
Direct amide formation from unactivated carboxylic acids and amines
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
가장 최신 버전 중 하나를 선택하세요:
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
![Dichloro[rac-ethylenebis(4,5,6,7-tetrahydro-1-indenyl)]zirconium(IV) 97%](/deepweb/assets/sigmaaldrich/product/structures/571/886/f2860a8d-a40a-461c-ba1e-93913a644d32/640/f2860a8d-a40a-461c-ba1e-93913a644d32.png)
![Dichloro[rac-ethylenebis(indenyl)]zirconium(IV)](/deepweb/assets/sigmaaldrich/product/structures/296/699/b249f923-58d9-45b3-bd63-b110942453d3/640/b249f923-58d9-45b3-bd63-b110942453d3.png)