모든 사진(2)
About This Item
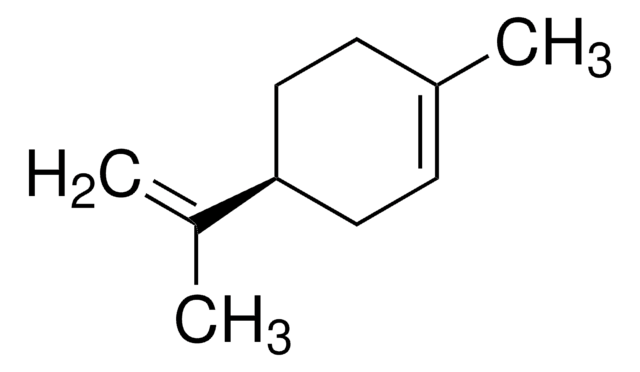
실험식(Hill 표기법):
C10H14O2
CAS Number:
Molecular Weight:
166.22
MDL number:
UNSPSC 코드:
12352002
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
95%
형태
flakes
광학 활성
[α]20/D −102°, c = 2 in methanol
mp
129-131 °C (lit.)
저장 온도
2-8°C
SMILES string
CC(=C)[C@H]1CCC(=CC1)C(O)=O
InChI
1S/C10H14O2/c1-7(2)8-3-5-9(6-4-8)10(11)12/h5,8H,1,3-4,6H2,2H3,(H,11,12)/t8-/m1/s1
InChI key
CDSMSBUVCWHORP-MRVPVSSYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
(S)-(-)-Perillic acid is a perillyl derivative with potent antimicrobial and anticancer activity. It can be prepared via bio-oxidation of R-(+)-limonene.
애플리케이션
(S)-(−)-Perillic acid (PA) can be used as a starting material for the preparation of:,·
- Aryl amides, 4-(prop-1-en-2-yl)-N-(3-(trifluoromethyl)phenyl)cyclohex-1-ene-1-carboxamide and N-(4-(4-amino-2-methylphenethyl)-3-methylphenyl)-4-(prop-1-en-2-yl)cyclohex-1-ene-1-carboxamide, as in vitro antiproliferative active agents.
- Machaeriols and cannabinoid-related compounds as antimalarial agents.
- Tricyclic-β-lactone by treating with 1-chloro-N,N-2-trimethylpropenylamine and dimethylketomalonate, followed by addition of 9-azajulolidine.
생화학적/생리학적 작용
Interferes with activity of p21ras and other small G proteins by inhibiting post-translational cysteine isoprenylation.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Natural product derivatization with β -lactones, β -lactams and epoxides toward `infinite?binders
Jouanneau M, et al.
Tetrahedron, 75(24), 3348-3354 (2019)
Novel N-arylamide derivatives of (S)-perillic acid ((S)-PA): in vitro and in vivo cytotoxicity and antitumor evaluation
Mukhtar YM, et al.
Royal Society of Chemistry Advances, 9(35), 19973-19982 (2019)
Felix Klotter et al.
Angewandte Chemie (International ed. in English), 54(29), 8547-8550 (2015-06-17)
Short and highly efficient stereoselective syntheses provide machaeriols and cannabinoids in a divergent approach starting from a common precursor, commercially available (S)-perillic acid. Key features of the novel strategy are a stereospecific palladium-catalyzed decarboxylative arylation and a one-pot sequence comprising
Bioconversion of R-(+)-limonene to perillic acid by the yeast Yarrowia lipolytica.
Ferrara MA, et al.
Brazilian Journal of Microbiology, 44(4), 1075-1080 (2013)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.