모든 사진(3)
About This Item
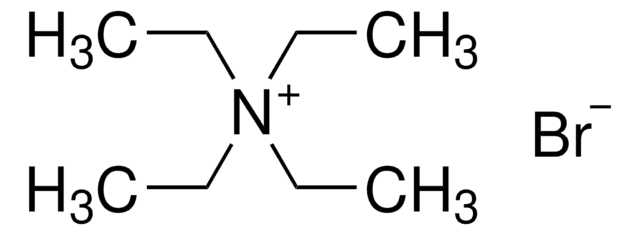
Linear Formula:
(CH3CH2CH2)4N(Br)
CAS Number:
Molecular Weight:
266.26
Beilstein:
3567846
EC Number:
MDL number:
UNSPSC 코드:
12352107
PubChem Substance ID:
NACRES:
NA.22
추천 제품
일반 설명
Tetrapropylammonium bromide (TPAB) is a quaternary ammonium salt. It is commonly used as a phase-transfer catalyst to facilitate charged species transfer between phases during organic synthesis and various chemical reactions. It also exhibits environmental compatibility, operational simplicity, non-corrosiveness, and ease of reusability, rendering it a suitable material for organic synthesis .
애플리케이션
Tetrapropylammonium bromide is used as a structure-directing agent in the synthesis of:
- ZSM-5 zeolite, which is a major catalyst in the petroleum and fine chemical industries.
- Microporous and mesoporous materials that have potential applications in electronics (conducting polymers) and catalysis.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
A Facile Synthesis of Xanthene-1, 8 (2H)-dione Derivatives by Using Tetrapropylammonium Bromide as Catalyst
Poursattar Marjani A, et al.
Journal of Heterocyclic Chemistry, 55(6), 1324-1330 (2018)
Hui Wang et al.
Physical chemistry chemical physics : PCCP, 17(32), 20636-20646 (2015-07-24)
The (13)C NMR chemical shift moving upfield indicates the main model of π-holeX(-) bond between cyanuric chloride/1,3,5-triazine (3ClN/3N), which possess both the π-hole and σ-hole, and X(-). (13)C NMR and UV absorption titration in acetonitrile confirmed that the bonding abilities
Yong Peng et al.
Angewandte Chemie (International ed. in English), 54(19), 5709-5712 (2015-03-19)
The fabrication of MFI zeolite films with particular b-axis orientation is especially fascinating. Unlike the conventional alkaline or hydrofluoric acid (HF) assisted neutral synthesis route, here we develop a novel neutral synthesis solution system of TPABr/fumed silica/H2 O without the
Sarika Goel et al.
Journal of the American Chemical Society, 136(43), 15280-15290 (2014-10-15)
The encapsulation of metal clusters (Pt, Ru, Rh) within MFI was achieved by exchanging cationic metal precursors into a parent zeolite (BEA, FAU), reducing them with H2 to form metal clusters, and transforming these zeolites into daughter structures of higher
Joel G Davis et al.
The journal of physical chemistry. B, 119(29), 9417-9422 (2014-11-22)
Raman multivariate curve resolution (Raman-MCR), as well as quantum and classical calculations, are used to probe water structural changes in the hydration shells of carboxylic acids and tetraalkyl ammonium ions with various aliphatic chain lengths. The results reveal that water
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.