237817
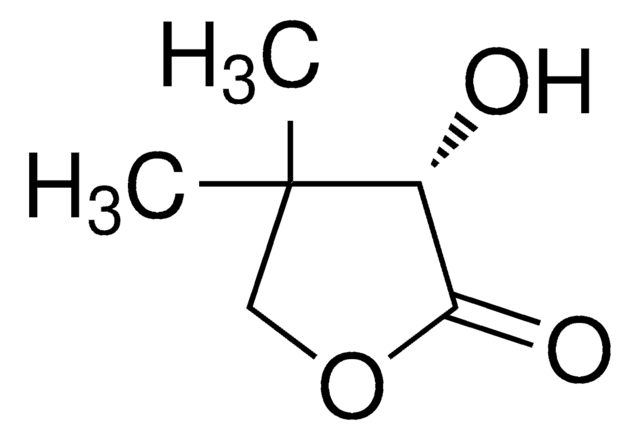
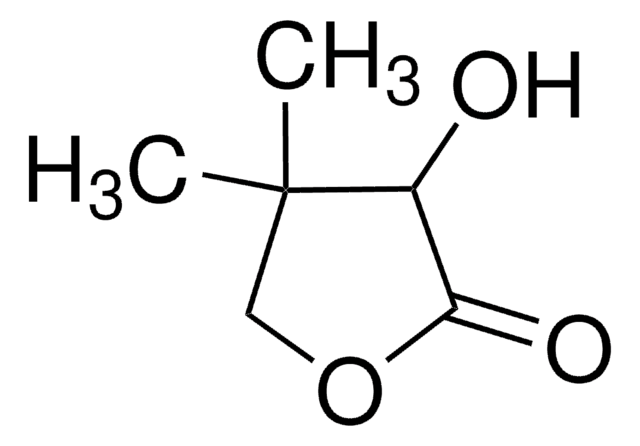
D-(−)-Pantolactone
99%
동의어(들):
(R)-(−)-α-Hydroxy-β,β-dimethyl-γ-butyrolactone, (R)-(−)-β,β-Dimethyl-α-hydroxy-γ-butyrolactone, (R)-(−)-Pantolactone, Pantoic acid γ-lactone
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
실험식(Hill 표기법):
C6H10O3
CAS Number:
Molecular Weight:
130.14
Beilstein:
80957
EC Number:
MDL number:
UNSPSC 코드:
12352005
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
99%
양식
solid
광학 활성
[α]25/D −49.8°, c = 2 in H2O
bp
120-122 °C/15 mmHg (lit.)
mp
91 °C (lit.)
작용기
ester
hydroxyl
SMILES string
CC1(C)COC(=O)[C@@H]1O
InChI
1S/C6H10O3/c1-6(2)3-9-5(8)4(6)7/h4,7H,3H2,1-2H3/t4-/m0/s1
InChI key
SERHXTVXHNVDKA-BYPYZUCNSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
D-(-)-Pantolactone is a chiral auxiliary used in many asymmetric synthesis reactions.
It can be used as a chiral starting material to synthesize:
It can be used as a chiral starting material to synthesize:
- An insect sex pheromone named 1S,2S,3R-1-acetoxymethyl-2,3,4,4-tetrameth-ylcyclopentane.
- (-)-Enantiomer of (R)-8-hydroxy-4,7,7-trimethyl-7,8-dihydrocyclopenta[e]isoindole-1,3(2H,6H)-dione, a norsesquiterpene alkaloid.
- A bicyclic diterpene named isofregenedadiol.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Enantiospecific synthesis of sex pheromone of the obscure mealybug from pantolactone via tandem conjugate addition/cyclization
Hajare AK, et al.
Tetrahedron Letters, 51(40), 5291-5293 (2010)
Asymmetric diels alder reactions and michael type additions with 6 (R)-3′(R)-Pantolactone-substituted-2H-pyran-3 (6H)-one.
Knol J, et al.
Tetrahedron Letters, 32(50), 7465-7468 (1991)
Total synthesis of isofregenedadiol
Kurhade SE, et al.
Organic Letters, 13(14), 3690-3693 (2011)
Total synthesis of an anticancer norsesquiterpene alkaloid isolated from the fungus Flammulina velutipes
Kashinath K, et al.
Organic & Biomolecular Chemistry, 12(24), 4098-4103 (2014)
Matthew B Kubilius et al.
ACS omega, 2(11), 8308-8312 (2017-11-21)
1-Ethyl-3-[3-dimethylaminopropyl]carbodiimide (EDC) is a commonly used reagent for bioconjugation and peptide synthesis. Both EDC and the corresponding urea derivative, 1-(3-dimethylaminopropyl)-3-ethylurea (EDU), are achiral. As the reagent is active in aqueous solutions, it is a common choice for the study of
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.