추천 제품
분석
97%
형태
liquid
refractive index
n20/D 1.614 (lit.)
bp
143 °C/34 mmHg (lit.)
mp
−80 °C (lit.)
density
1.052 g/mL at 25 °C (lit.)
SMILES string
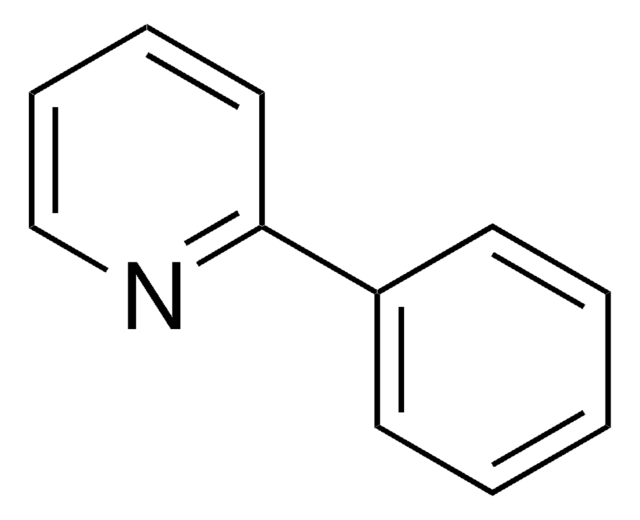
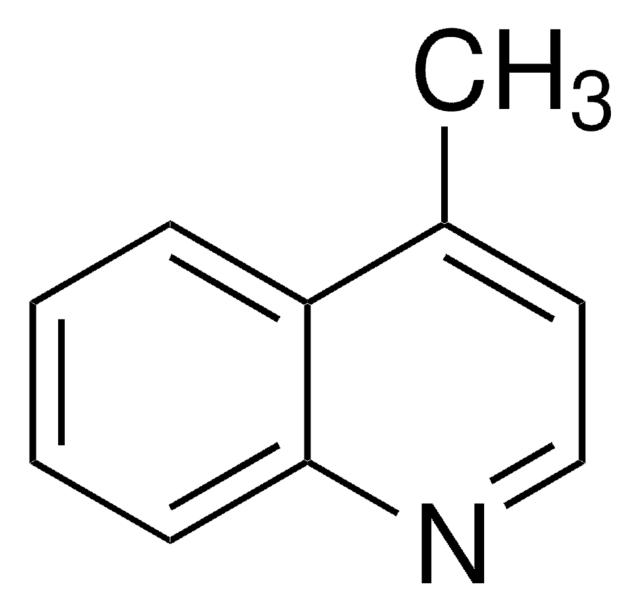
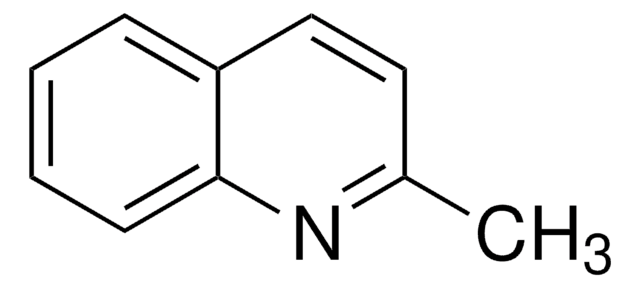
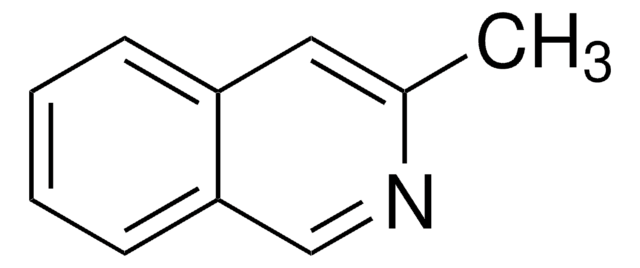
Cc1cccc2cccnc12
InChI
1S/C10H9N/c1-8-4-2-5-9-6-3-7-11-10(8)9/h2-7H,1H3
InChI key
JRLTTZUODKEYDH-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Tumorigenic potential of 8-methylquinoline has been evaluated in newborn CD-1 mice and Sprague-Dawley rats.
애플리케이션
8-Methylquinoline has been used in preparation of osmium chloridophosphine complexes, as quinoline carbene tautomers.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
221.0 °F - closed cup
Flash Point (°C)
105 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Kate B McMurtrey et al.
Organic letters, 14(16), 4094-4097 (2012-08-01)
The palladium-catalyzed C-H fluorination of 8-methylquinoline derivatives with nucleophilic fluoride is reported. This transformation involves the use of AgF as the fluoride source in combination with a hypervalent iodine oxidant. Both the scope and mechanism of the reaction are discussed.
Synthetic routes to N-heterocyclic carbene complexes: pyridine-carbene tautomerizations.
Doris Kunz
Angewandte Chemie (International ed. in English), 46(19), 3405-3408 (2007-04-05)
Kami L Hull et al.
Journal of the American Chemical Society, 128(22), 7134-7135 (2006-06-01)
This communication describes the development of a new Pd-catalyzed method for the fluorination of carbon-hydrogen bonds. A key step of these transformations involves palladium-mediated carbon-fluorine coupling-a much sought after, but previously unprecedented, transformation. These reactions were successfully achieved under oxidative
R Yang et al.
Luminescence : the journal of biological and chemical luminescence, 16(2), 129-133 (2001-04-20)
8-Methylquinoline is unique among the monomethylquinolines in the red-shift it shows in the absorption band derived from the short axis polarized ((1)L(a) <-- (1)A) electronic transition, relative to that in quinoline itself. The effect is even more pronounced in the
C E Scharping et al.
Carcinogenesis, 14(5), 1041-1047 (1993-05-01)
The hepatic microsomal metabolism of the carcinogenic 8-methylquinoline (8MQ) and its noncarcinogenic isomer, 6-methylquinoline (6MQ), were compared for preparations from control rats and rats pretreated with phenobarbital or 3-methylcholanthrene. For each compound the alcohol was the major metabolite, constituting 50-75%
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.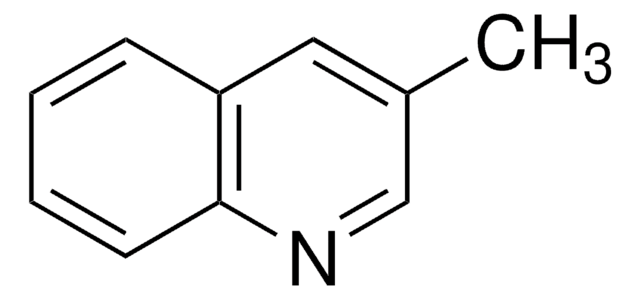

![Benzo[h]quinoline 97%](/deepweb/assets/sigmaaldrich/product/structures/344/715/928932d2-4ca4-4402-b56c-85a80100ce17/640/928932d2-4ca4-4402-b56c-85a80100ce17.png)