263567
1,4-Bis(trimethylsilyl)butadiyne
98%, stable crystalline form of butadiyne
동의어(들):
1,4-Bis(trimethylsilyl)-1,3-butadiyne, BTMSBD
로그인조직 및 계약 가격 보기
모든 사진(3)
About This Item
Linear Formula:
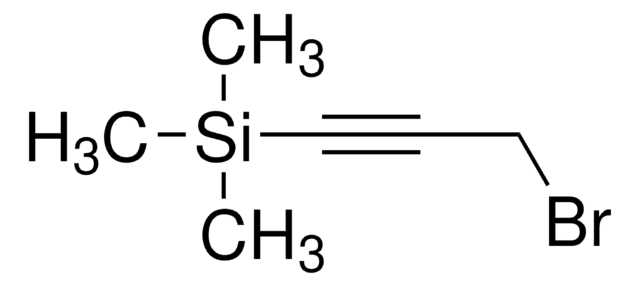
(CH3)3SiC≡CC≡CSi(CH3)3
CAS Number:
Molecular Weight:
194.42
Beilstein:
1758268
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
98%
형태
solid
refractive index
n20/D 1.384 (lit.)
mp
111-113 °C (lit.)
density
0.974 g/mL at 20 °C (lit.)
저장 온도
2-8°C
SMILES string
C[Si](C)(C)C#CC#C[Si](C)(C)C
InChI
1S/C10H18Si2/c1-11(2,3)9-7-8-10-12(4,5)6/h1-6H3
InChI key
LBNVCJHJRYJVPK-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
1,4-Bis(trimethylsilyl)butadiyne can be used as a reagent to prepare:
- 1,1,3,4-Tetrasilyl-substituted 1,3-butadienes or 1,1,3,4-tetrasilyl-substituted 1,2-butadienes by hydrosilylation reaction using various hydridosilanes and catalysts.
- Glycosylated oligo(ethynylene)s using the Negishi reaction.
- (±) Falcarinol, a polyacetylene class of fatty alcohol.
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
Tobias N Hoheisel et al.
Organic letters, 10(20), 4525-4528 (2008-09-25)
A convenient and efficient sp-sp carbon heterocoupling protocol based on the Negishi reaction was developed, in which the required zinc diacetylide was generated from 1,4-bis(trimethylsilyl)butadiyne in situ and reacted with a bromoacetylene in apolar solvent mixtures. The method has been
A convenient Negishi protocol for the synthesis of glycosylated oligo (ethynylene) s
Hoheisel TN and Frauenrath H
Organic Letters, 10, 4525-4528 (2008)
A short synthesis of (+) and (-)-falcarinol
McLaughlin NP, et al.
Tetrahedron, 66, 9681-9687 (2010)
Hydrosilylation of 1, 4-Bis (trimethylsilyl) butadiyne and Silyl-Substituted Butenynes.
Kusumoto T, et al.
Bulletin of the Chemical Society of Japan, 65(5), 1280-1290 (1992)
Manuel M Bentlohner et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 23(67), 17089-17094 (2017-09-15)
We report on the synthesis and structure, as well as on the mechanism of formation of the [Ge
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.





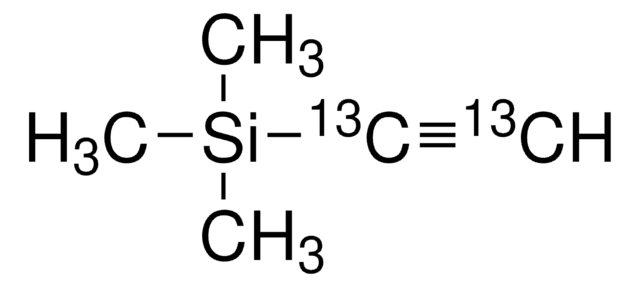

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)