추천 제품
Quality Level
분석
99%
양식
liquid
refractive index
n20/D 1.626 (lit.)
bp
103 °C/1 mmHg (lit.)
density
1.165 g/mL at 25 °C (lit.)
SMILES string
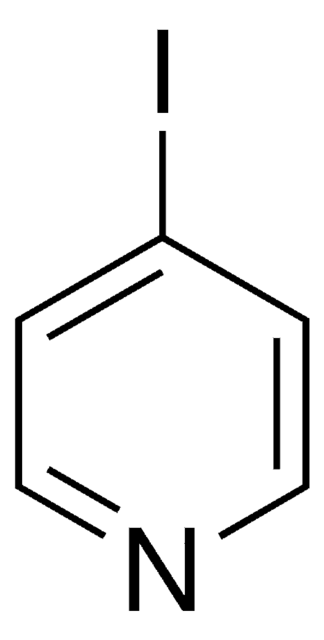
c1ccn2ccnc2c1
InChI
1S/C7H6N2/c1-2-5-9-6-4-8-7(9)3-1/h1-6H
InChI key
UTCSSFWDNNEEBH-UHFFFAOYSA-N
관련 카테고리
일반 설명
In vivo anti-trypanosomal activity of imidazo[1,2-a]pyridiness in the STIB900 mouse model has been investigated.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
235.4 °F - closed cup
Flash Point (°C)
113 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Hongpeng Sun et al.
The Journal of organic chemistry, 77(23), 10745-10751 (2012-11-22)
An efficient tandem route to the synthesis of 3H-1,2a(1),3-triazaacenaphthylene derivatives of the cyclazine family has been developed. Target compounds were obtained in moderate to good yields by a Yb(OTf)(3)/Ag(2)CO(3)-catalyzed, three-component domino reaction. This in turn will set the stage for
Garrett C Moraski et al.
Bioorganic & medicinal chemistry, 20(7), 2214-2220 (2012-03-07)
Tuberculosis (TB) is a devastating disease resulting in a death every 20s. Thus, new drugs are urgently needed. Herein we report ten classes of compounds-oxazoline, oxazole, thiazoline, thiazole, pyrazole, pyridine, isoxazole, imidazo[1,2-a]pyridine, imidazo[1,2-a]pyrimidine and imidazo[1,2-c]pyrimidine-which have good (micromolar) to excellent
Richard Ducray et al.
Bioorganic & medicinal chemistry letters, 21(16), 4702-4704 (2011-07-19)
Following the discovery of imidazopyridine 1 as a potent IGF-1R tyrosine kinase inhibitor, the aniline part has been modified with the aim to optimize the properties of this series. The structure-activity relationships against IGF-1R kinase activity as well as inhibition
Mohamed A Ismail et al.
Bioorganic & medicinal chemistry, 16(2), 683-691 (2007-11-03)
The key dinitrile intermediates 4a-d were synthesized by reaction of phenacyl bromide 1 and the appropriate 2-amino-5-bromopyridines to yield 3a-d. Suzuki coupling of 3a-d with 4-cyanophenylboronic acid yielded the 2,6-bis(4-cyanophenyl)-imidazo[1,2-a]pyridine derivatives 4a-d. The bis-amidoximes 5a-d, obtained from 4a-d by the
Suren Husinec et al.
Organic letters, 13(9), 2286-2289 (2011-03-31)
A base promoted cyclization of the protected N-propargylaminopyridines was shown to be an efficient method for the preparation of imidazo[1,2-a]pyridine derivatives. The reactions were carried out with a small excess of base, at room temperature or slightly above producing the
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.![2-phenylimidazo[1,2-a]pyridine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/281/247/6c2550a0-2f0c-4866-83d8-3c1fb039e165/640/6c2550a0-2f0c-4866-83d8-3c1fb039e165.png)
![Imidazo[1,2-a]pyrazine 97%](/deepweb/assets/sigmaaldrich/product/structures/370/804/1712d71f-52fb-4758-9a22-85b6c96cd4e8/640/1712d71f-52fb-4758-9a22-85b6c96cd4e8.png)
![Imidazo[1,2-a]pyrimidine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/187/001/4862c14e-bec7-4475-85a5-f178e48ff60f/640/4862c14e-bec7-4475-85a5-f178e48ff60f.png)