모든 사진(1)
About This Item
실험식(Hill 표기법):
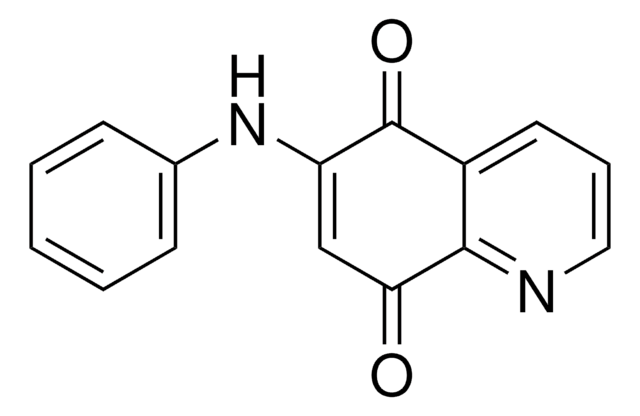
C13H7NO2
CAS Number:
Molecular Weight:
209.20
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
99%
mp
178-180 °C (lit.)
작용기
ketone
SMILES string
O=C1c2ccccc2C(=O)c3cnccc13
InChI
1S/C13H7NO2/c15-12-8-3-1-2-4-9(8)13(16)11-7-14-6-5-10(11)12/h1-7H
InChI key
ZLLVUAAESHIVAZ-UHFFFAOYSA-N
일반 설명
Benz[g]isoquinoline-5,10-dione has been isolated as an active component from the ethanolic extract of the aerial parts of Mitracarpus scaber. It exhibits significant in vitro inhibitory activity against the AIDS-related pathogens. The in vitro antibacterial and anti-fungal activity of benz[g]isoquinoline-5,10-dione has been investigated using the agar well-diffusion assay.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
가장 최신 버전 중 하나를 선택하세요:
A M Clark et al.
Pharmaceutical research, 1(6), 269-271 (1984-11-01)
The in vitro antibacterial and anti-fungal activity of benz[g]isoquinoline-5,10-dione (1), benzo[g]quinoline-5, 10-dione (2), benzo[g]quinoline-5,6-dione (3), and anthraquinone (4) was determined using the agar well-diffusion assay. The minimum inhibitory concentrations (MIC's) of each of the active compounds (1-3) was determined using
A L Okunade et al.
Planta medica, 65(5), 447-448 (1999-07-27)
An ethanolic extract of the aerial parts of Mitracarpus scaber demonstrated good antimicrobial activity. Bioassay directed fractionation of this extract led to the isolation of benz[g]isoquinoline-5,10-dione (1) as an active component. Compound 1 showed significant in vitro inhibitory activity against
Ekaterina Shinkevich et al.
Organic & biomolecular chemistry, 9(2), 538-548 (2010-10-27)
1,2-Disubstituted 1,2,3,4-tetrahydrobenz[g]isoquinoline-5,10-diones are prepared for the first time through an activated Pictet-Spengler reaction of the corresponding imines of 2-(1,4-dimethoxynaphth-2-yl)ethylamine in the presence of an acyl chloride and AlCl(3) followed by an oxidation with silver(II) oxide in nitric acid. Depending on
B T Walton et al.
Science (New York, N.Y.), 222(4622), 422-423 (1983-10-28)
Morphological abnormalities including extra compound eyes, extra heads, and distally duplicated legs were generated in cricket embryos by treating eggs with single doses of either benz[g]isoquinoline-5,10-dione or benzo[h]quinoline-5,6-dione. Slight structural modifications of the molecules resulted in a loss of teratogenic
Andrew Jonathan Nok
Cell biochemistry and function, 20(3), 205-212 (2002-07-19)
An ethanolic extract of Mitracarpus scaber was found to possess in vitro and in vivo trypanocidal activity against Trypanosoma congolense. At a dosage of 50 mg kg(-1) day(-1) in normal saline for 5 days, the extract cured Balbc mice infected
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
![1H-Benzo[g]indole 97%](/deepweb/assets/sigmaaldrich/product/structures/568/798/abc69b41-4c75-4dce-8e3a-b6ff7851c6fd/640/abc69b41-4c75-4dce-8e3a-b6ff7851c6fd.png)