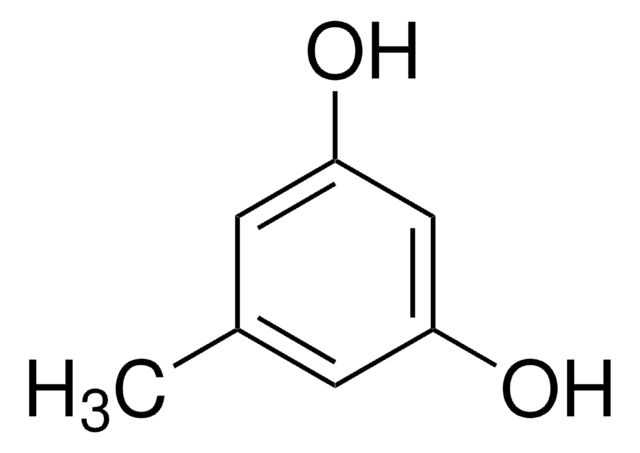
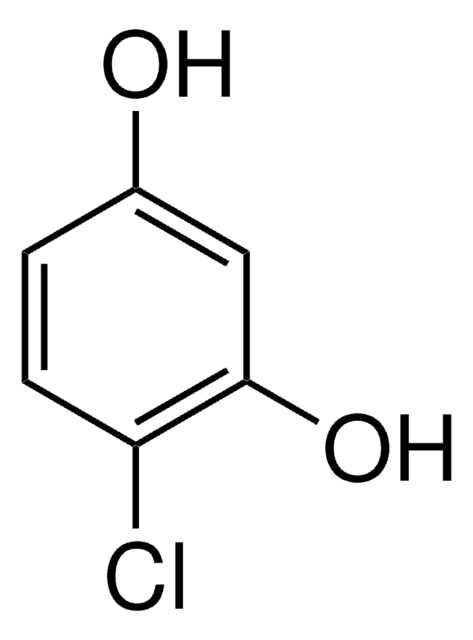
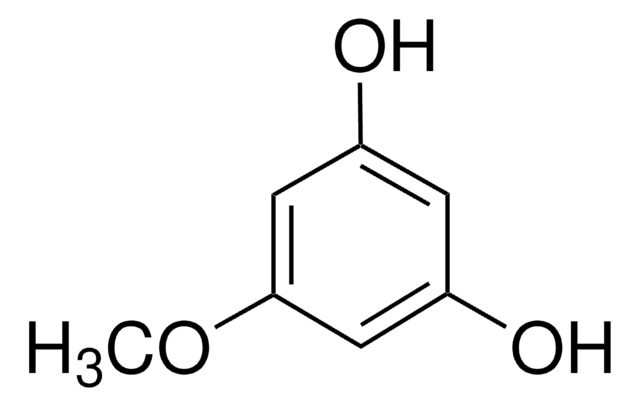
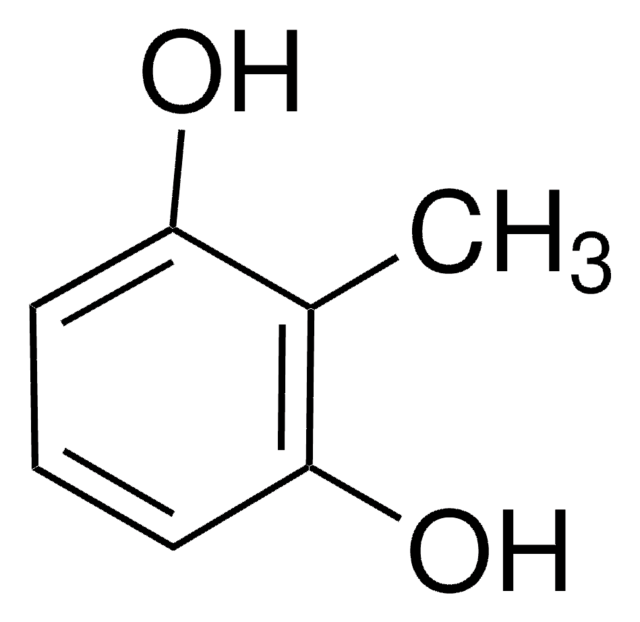
추천 제품
일반 설명
The reaction between 2-methylresorcinol and 2-alkenals was studied to investigate the scavenging ability of m-diphenols for the 2-alkenals formed during lipid oxidation.
애플리케이션
2-Methylresorcinol was used in the synthesis of:
- C-5-bromo-2-hydroxyphenylcalix[4]-2-methylresorcinarene
- tripyrrane analogs
- series of novel aromatic benziporphyrins
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Acute 1 - Eye Dam. 1 - Skin Sens. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
275.0 °F - closed cup
Flash Point (°C)
135 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
이미 열람한 고객
Hamza M Abosadiya et al.
Molecules (Basel, Switzerland), 18(11), 13369-13384 (2013-11-01)
C-5-bromo-2-hydroxyphenylcalix[4]-2-methylresorcinarene (I) was synthesized by cyclocondensation of 5-bromo-2-hydroxybenzaldehyde and 2-methylresorcinol in the presence of concentrated HCl. Compound I was characterized by infrared and nuclear magnetic resonance spectroscopic data. X-ray analysis showed that this compound crystallized in a triclinic system with
Kae Miyake et al.
Chemical communications (Cambridge, England), (2)(2), 178-179 (2004-01-23)
Acid catalyzed condensation of resorcinol or 2-methylresorcinol with 2 equiv. of an acetoxymethylpyrrole gave bis(pyrrolylmethyl)benzene derivatives in moderate yields; these afforded a series of novel aromatic benziporphyrins using the MacDonald "3 + 1" methodology.
Francisco J Hidalgo et al.
Food chemistry, 160, 118-126 (2014-05-07)
The reaction between m-diphenols (resorcinol, 2-methylresorcinol, 2,5-dimethylresorcinol, 3-methylphenol, orcinol, and phloroglucinol) and 2-alkenals (2-pentenal and 2-octenal) was studied in an attempt to understand the chemical pathways involved in the scavenging ability of m-diphenols for the 2-alkenals produced as a consequence
Timothy D Lash et al.
The Journal of organic chemistry, 76(15), 6295-6308 (2011-06-23)
Tripyrrane analogues were prepared by reacting resorcinol or 2-methylresorcinol with 2 equiv of an acetoxymethylpyrrole in the presence of p-toluenesulfonic acid and calcium chloride. Following removal of the benzyl ester protective groups, the resorcinol-derived benzitripyrrane was reacted with a pyrrole
O K Davydova et al.
Mikrobiologiia, 75(5), 662-669 (2006-11-10)
The fact of long-term preservation of the physicochemical properties of DNA molecules in aqueous solutions in complexes with methylresorcinol, hexylresorcinol, and tyrosol, the chemical analogues of microbial autoregulators (d1 factors) from the group of alkylhydroxybenzenes (AOB), was established. Compared to
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.