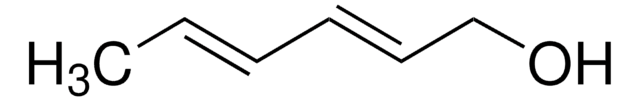
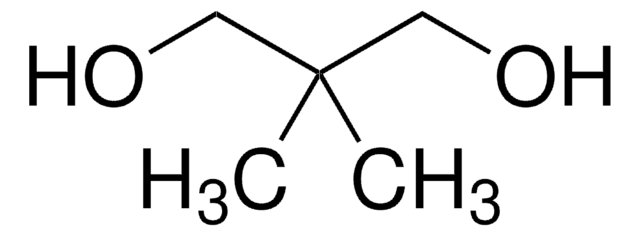
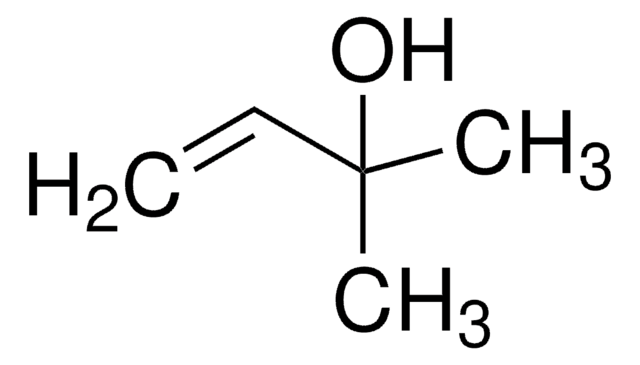
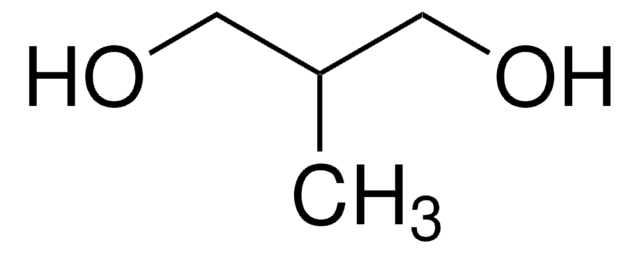
추천 제품
분석
≥96%
형태
liquid
포함
0.4% hydroquinone as stabilizer
refractive index
n20/D 1.445 (lit.)
bp
115-116 °C (lit.)
density
0.865 g/mL at 25 °C (lit.)
SMILES string
OC(C=C)C=C
InChI
1S/C5H8O/c1-3-5(6)4-2/h3-6H,1-2H2
InChI key
ICMWSAALRSINTC-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Starting material for asymmetric epoxidation.
Substrate employed in a synthesis of amino-substituted dienes via a bismuth-catalyzed SNi displacement of alcohols by sulfonamide nucleophiles.
Useful building block in natural product synthesis.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
89.6 °F - closed cup
Flash Point (°C)
32 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Masanori Imai et al.
The Journal of organic chemistry, 69(4), 1144-1150 (2004-02-14)
Intermolecular hydroacylation between salicylaldehydes 1, 26-40 and 1,4-penta- or 1,5-hexadienes 4-13 by Rh-catalyst proceeded under mild reaction conditions to give a mixture of iso- and normal-hydroacylated products 14-25, 41-55, and 57-60. In the hydroacylation reaction, chelation of both salicylaldehyde and
Tetrahedron Asymmetry, 4, 1533-1533 (1993)
P Andrew Evans et al.
Journal of the American Chemical Society, 125(48), 14702-14703 (2003-12-04)
The enantioselective total synthesis of the annonaceous acetogenin (-)-mucocin (1) was accomplished using a triply convergent 12-step sequence (longest linear sequence) in 13.6% overall yield. This represents the first application of the temporary silicon-tethered (TST) ring-closing metathesis (RCM) cross-coupling reaction
Chin. J. Chem., 9, 381-381 (1991)
Bismuth-catalyzed direct substitution of the hydroxy group in alcohols with sulfonamides, carbamates, and carboxamides.
Hongbo Qin et al.
Angewandte Chemie (International ed. in English), 46(3), 409-413 (2006-12-06)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.