모든 사진(1)
About This Item
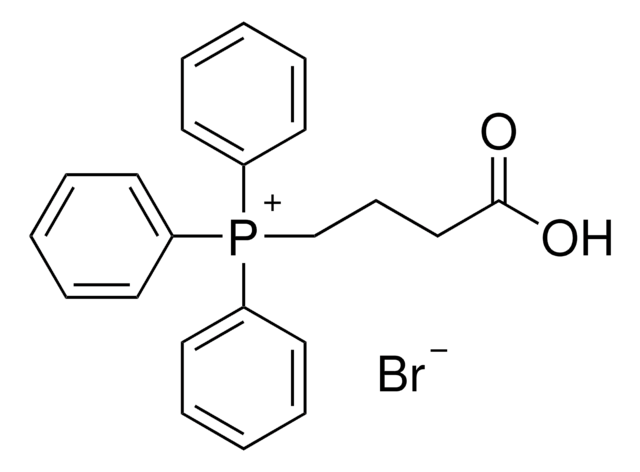
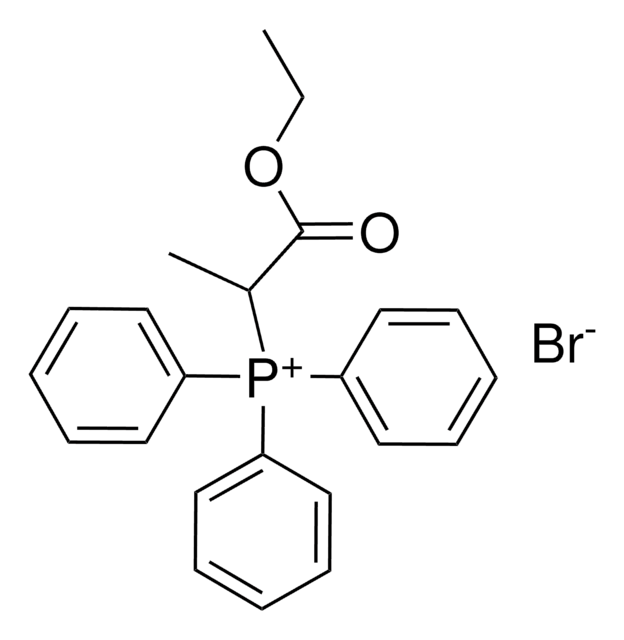
Linear Formula:
C2H5O2C(CH2)3P(C6H5)3Br
CAS Number:
Molecular Weight:
457.34
MDL number:
UNSPSC 코드:
12352108
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
97%
반응 적합성
reaction type: C-C Bond Formation
mp
165-167 °C (lit.)
작용기
ester
phosphine
SMILES string
[Br-].CCOC(=O)CCC[P+](c1ccccc1)(c2ccccc2)c3ccccc3
InChI
1S/C24H26O2P.BrH/c1-2-26-24(25)19-12-20-27(21-13-6-3-7-14-21,22-15-8-4-9-16-22)23-17-10-5-11-18-23;/h3-11,13-18H,2,12,19-20H2,1H3;1H/q+1;/p-1
InChI key
JPZMNVPVVYVXAD-UHFFFAOYSA-M
애플리케이션
Reactant for:
- Asymmetric synthesis of antihypercholesterolemic ezetimibe via Wittig reaction
- Wittig reactions
[3-(Ethoxycarbonyl)propyl]triphenylphosphonium bromide can be used:
- As a reactant in the synthesis of spirocyclic GPR119 agonists.
- In the synthesis of cyclic acetal intermediate of azadirachtin, a terpenoid found in the neem tree Azadirachta indica.
- To prepare a catechin metabolite named 5-(3,4,5-trihydroxyphenyl)valeric acid.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
가장 최신 버전 중 하나를 선택하세요:
Inhibitory activity of catechin metabolites produced by intestinal microbiota on proliferation of HeLa cells
Hara-Terawaki A, et al.
Biological & Pharmaceutical Bulletin, 40(8), 1331-1335 (2017)
Synthetic studies of azadirachtin. Synthesis of the cyclic acetal intermediate in the naturally occurring form
Nishikimi Y, et al.
The Journal of Organic Chemistry, 54(14), 3354-3359 (1989)
Design and synthesis of novel and potent GPR119 agonists with a spirocyclic structure
Harada K, et al.
Bioorganic & medicinal chemistry letters, 28(7), 1228-1233 (2018)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.