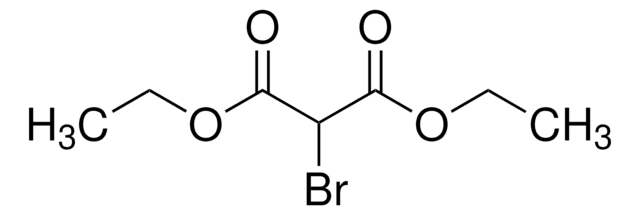
추천 제품
Grade
technical grade
Quality Level
분석
90%
양식
liquid
refractive index
n20/D 1.460 (lit.)
bp
105-108 °C/11 mmHg (lit.)
density
1.601 g/mL at 25 °C (lit.)
작용기
bromo
ester
SMILES string
COC(=O)C(Br)C(=O)OC
InChI
1S/C5H7BrO4/c1-9-4(7)3(6)5(8)10-2/h3H,1-2H3
InChI key
NEMOJKROKMMQBQ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Dimethyl bromomalonate undergoes manganese(III)-promoted free-radical chain addition reaction with olefins, to yield dimethyl 2-bromoalkylmalonates. Organocatalyzed Michael addition of dimethyl bromomalonate to nitrostyrenes to yield synthetically useful nitrocyclopropanes has been reported. Dimethyl bromomalonate reacts readily with arylnitroso compounds to yield the corresponding N-aryl-C,C-dimethoxycarbonylnitrones.
애플리케이션
Dimethyl bromomalonate may be used in the enantioselective synthesis of nitrocyclopropanes.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
230.0 °F - closed cup
Flash Point (°C)
110 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
Synthesis and 1, 3-dipolar cycloaddition reactions of N-aryl-C, C-dimethoxycarbonylnitrones.
Tomioka Y, et al.
Journal of Heterocyclic Chemistry, 40(1), 121-128 (2003)
Stereoselective synthesis of functionalised cyclopropanes from nitroalkenes via an organocatalysed Michael-initiated ring-closure approach.
Russo A and Lattanzi A.
Tetrahedron Asymmetry, 21(9), 1155-1157 (2010)
Manganese (III)-promoted free-radical addition of dimethyl bromomalonate to olefins using an electrochemical regeneration procedure.
Nedelec JY and Nohair K.
Synlett, 09, 659-660 (1991)
Yi-Ning Xuan et al.
Organic letters, 11(7), 1583-1586 (2009-03-06)
Highly enantioselective synthesis of nitrocyclopropanes was achieved via the organocatalytic conjugate addition of dimethyl bromomalonate to nitroalkenes and the consequent intramolecular cyclopropanation. 6'-Demethyl quinine was found to be the efficient catalyst. Excellent enantioselectivities, diastereoselectivities, and good yields were obtained for
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.