모든 사진(1)
About This Item
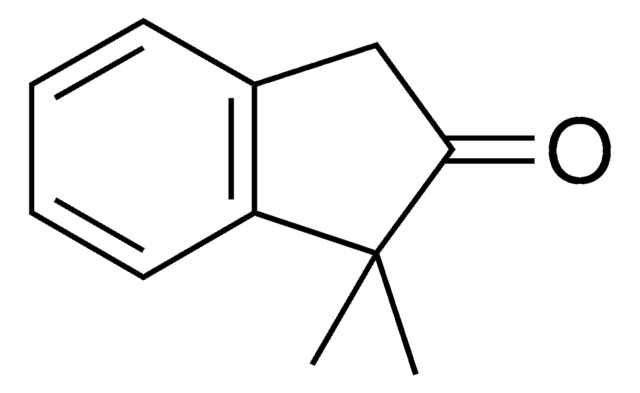
실험식(Hill 표기법):
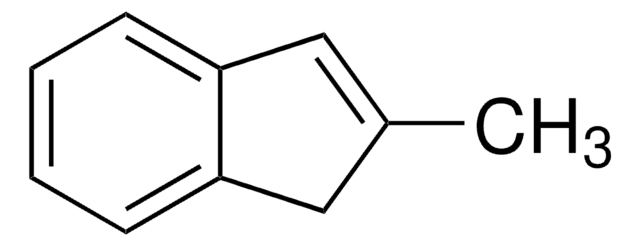
C10H10O
CAS Number:
Molecular Weight:
146.19
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
99%
형태
liquid
refractive index
n20/D 1.555 (lit.)
bp
93-95 °C/4 mmHg (lit.)
density
1.064 g/mL at 25 °C (lit.)
작용기
ketone
SMILES string
CC1Cc2ccccc2C1=O
InChI
1S/C10H10O/c1-7-6-8-4-2-3-5-9(8)10(7)11/h2-5,7H,6H2,1H3
InChI key
BEKNOGMQVKBMQN-UHFFFAOYSA-N
일반 설명
2-Methyl-1-indanone, a α-benzocycloalkenone, is a derivative of 1-indanone. Its synthesis has been reported. The enzymatic dynamic kinetic resolution (DKR) of racemic 2-methyl-1-indanone has been studied. The asymmetric α-arylation and hydroxymethylation of 2-methyl-1-indanone has been reported. It participated in the synthesis of 2-methyl-6-carboxyazulene.
애플리케이션
2-Methyl-1-indanone may be used as a starting material in the synthesis of β-benzocycloalkenone. It may be used in the synthesis of the following:
- cyclohex-2-en-1-yl 2-methyl-1H-inden-3-yl carbonate
- 2-hydroxy-2-methyl-1-indanone
- O-alkoxycarbonylation of lithium enolates
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves
Shaozhong Ge et al.
Journal of the American Chemical Society, 133(41), 16330-16333 (2011-09-16)
We report the α-arylation of ketones with a range of aryl chlorides with enantioselectivities from 90 to 99% ee catalyzed by the combination of Ni(COD)(2) and (R)-BINAP and the coupling of ketones with a range of heteroaryl chlorides with enantioselectivities
Michael W Justik et al.
Molecules (Basel, Switzerland), 10(1), 217-225 (2007-11-17)
The conversion of alpha-benzocycloalkenones to homologous beta-benzocyclo-alkenones containing six, seven and eight-membered rings is reported. This was accomplished via a Wittig olefination-oxidative rearrangement sequence using[hydroxy(tosyloxy)iodo]-benzene (HTIB) is the oxidant, that enables the synthesis of regioisomeric pairs of methyl-substituted beta-benzocycloalkenones. The
On the decarboxylation of 2-methyl-1-tetralone-2-carboxylic acid-oxidation of the enol intermediate by triplet oxygen.
Riahi A, et al.
New. J. Chem., 37(8), 2245-2249 (2013)
Michał Rachwalski et al.
Chemical Society reviews, 42(24), 9268-9282 (2013-09-26)
Deracemisation of racemic compounds is still the most important strategy to produce optically pure compounds despite many recent advances in asymmetric synthesis. Especially deracemisation approaches that give rise to single enantiomers are preferred, which can be achieved either by invoking
Taku Kitanosono et al.
Chemistry, an Asian journal, 10(1), 133-138 (2014-10-29)
Enzymes exhibit overwhelmingly superior catalysis compared with artificial catalysts. Current strategies to rival enzymatic catalysis require unmodified or minimally modified structures of active sites, gigantic molecular weight, and sometimes the use of harsh conditions such as extremely low temperatures in
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.