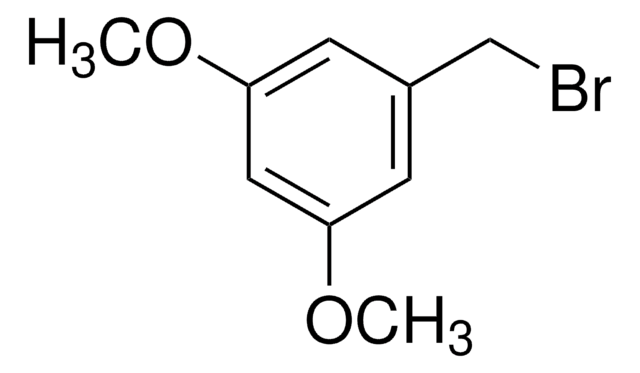
추천 제품
Quality Level
분석
98%
양식
liquid
refractive index
n20/D 1.575 (lit.)
bp
152 °C (lit.)
density
1.436 g/mL at 25 °C (lit.)
작용기
bromo
SMILES string
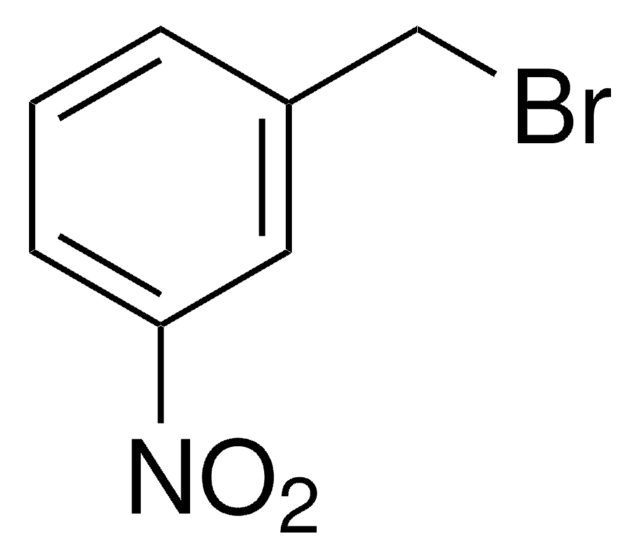
COc1cccc(CBr)c1
InChI
1S/C8H9BrO/c1-10-8-4-2-3-7(5-8)6-9/h2-5H,6H2,1H3
InChI key
ZKSOJQDNSNJIQW-UHFFFAOYSA-N
일반 설명
3-Methoxybenzyl bromide is a benzyl bromide derivative.
애플리케이션
3-Methoxybenzyl bromide (1-bromomethyl-3-methoxybenzene) may be used in the diastereoselective alkylation of vicinal dianions of chiral succinic acid derivatives.
It may be used in the synthesis of the following:
It may be used in the synthesis of the following:
- 6-(3-methoxyphenyl)-hexane-2,4-dione
- N-(3-methoxybenzyl)-N-(1-methyl-1-phenylethyl)-amine
- 2-(3-methoxybenzyl)-3-[(1R)-7,7-dimethyl-2-oxobicyclo[2.2.1]hept-1-yl]-(3S)-2-thionia-bicyclo [2.2.1]- heptane tetrafluoroborate
- 1-(3-methoxybenzyl)-5-(1-methyl-1H-imidazol-5-yl)-1H-1,2,3-triazole
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
235.4 °F - closed cup
Flash Point (°C)
113 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
Application of sulfur ylide mediated epoxidations in the asymmetric synthesis of ?-hydroxy-d-lactones. Synthesis of a mevinic acid analogue and (+)-prelactone B.
Aggarwal VK, et al.
Tetrahedron Asymmetry, 60(43), 9725-9733 (2004)
Synthesis of (-)-kainic acid using chiral lithium amides in an asymmetric dearomatizing cyclization.
Clayden J, et al.
Tetrahedron, 58(23), 4727-4733 (2002)
Highly diastereoselective alkylation of vicinal dianions of chiral succinic acid derivatives: a new general strategy to (R)-?-arylmethyl-?-butyrolactones.
Pohmakotr M, et al.
Tetrahedron Letters, 45(22), 4315-4318 (2004)
Yang Zhang et al.
The Journal of organic chemistry, 71(12), 4516-4520 (2006-06-06)
A mild protocol for the conversion of beta-ketoesters and beta-diketones to carboxylic acids with use of CAN in CH3CN is described. The method is compatible with a number of functional groups, and can generate carboxylic acids under neutral conditions at
Johan R Johansson et al.
The Journal of organic chemistry, 76(7), 2355-2359 (2011-03-11)
An experimentally simple sequential one-pot RuAAC reaction, affording 1,5-disubstituted 1H-1,2,3-triazoles in good to excellent yields starting from an alkyl halide, sodium azide, and an alkyne, is reported. The organic azide is formed in situ by treating the primary alkyl halide
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.