467154
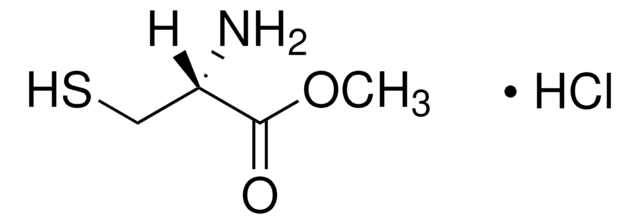
N-(tert-Butoxycarbonyl)-L-cysteine methyl ester
97%, for peptide synthesis
동의어(들):
N-Boc-L-cysteine methyl ester
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
HSCH2CH[NHCO2C(CH3)3]CO2CH3
CAS Number:
Molecular Weight:
235.30
MDL number:
UNSPSC 코드:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
추천 제품
product name
N-(tert-Butoxycarbonyl)-L-cysteine methyl ester, 97%
Quality Level
분석
97%
형태
liquid
광학 활성
[α]22/D +21°, c = 7.5 in chloroform
반응 적합성
reaction type: solution phase peptide synthesis
refractive index
n20/D 1.475 (lit.)
bp
214 °C (lit.)
density
1.143 g/mL at 25 °C (lit.)
응용 분야
peptide synthesis
SMILES string
COC(=O)[C@H](CS)NC(=O)OC(C)(C)C
InChI
1S/C9H17NO4S/c1-9(2,3)14-8(12)10-6(5-15)7(11)13-4/h6,15H,5H2,1-4H3,(H,10,12)/t6-/m0/s1
InChI key
NJGIAKIPSDCYAC-LURJTMIESA-N
애플리케이션
N-(tert-Butoxycarbonyl)-L-cysteine methyl ester is an N-terminal protected reagent that can be used in the synthesis of peptides and proteins containing cysteine residues, which can further be converted to dehydroalanine.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
235.4 °F - closed cup
Flash Point (°C)
113 °C - closed cup
이미 열람한 고객
Inactivation of NF-κB components by covalent binding of (−)-dehydroxymethylepoxyquinomicin to specific cysteine residues.
Yamamoto M, et al.
Journal of Medicinal Chemistry, 51(18), 5780-5788 (2008)
Methods for converting cysteine to dehydroalanine on peptides and proteins.
Chalker J M, et al.
Chemical Science, 2(9), 1666-1676 (2011)
Igor Linhart et al.
Archives of toxicology, 91(10), 3317-3325 (2017-03-12)
3-Nitrobenzanthrone (3-NBA), a potent environmental mutagen and carcinogen, is known to be activated in vivo to 3-benzanthronylnitrenium ion which forms both NH and C2-bound adducts with DNA and also reacts with glutathione giving rise to urinary 3-aminobenzanthron-2-ylmercapturic acid. In this
An insight into the radical thiol/yne coupling: the emergence of arylalkyne-tagged sugars for the direct photoinduced glycosylation of cysteine-containing peptides.
Minozzi M, et al.
The Journal of Organic Chemistry, 76(2), 450-459 (2010)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.