모든 사진(2)
About This Item
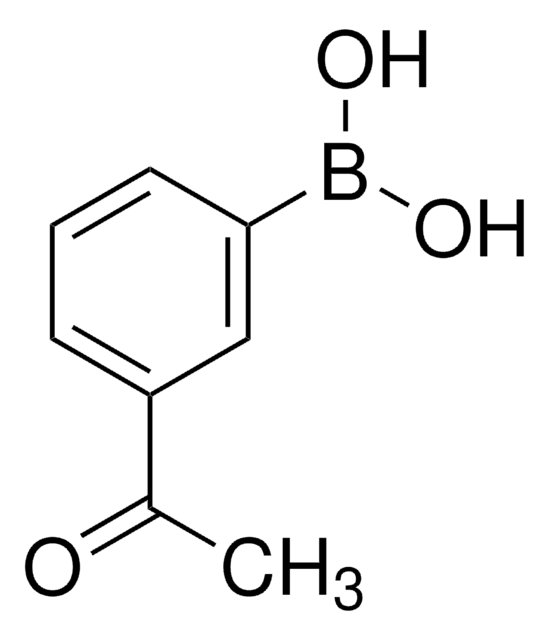
Linear Formula:
CH3COC6H4B(OH)2
CAS Number:
Molecular Weight:
163.97
MDL number:
UNSPSC 코드:
12352103
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
95%
mp
240-244 °C (lit.)
작용기
ketone
SMILES string
CC(=O)c1ccc(cc1)B(O)O
InChI
1S/C8H9BO3/c1-6(10)7-2-4-8(5-3-7)9(11)12/h2-5,11-12H,1H3
InChI key
OBQRODBYVNIZJU-UHFFFAOYSA-N
일반 설명
4-Acetylphenylboronic acid is a boronate, belongs to a class of synthetic organic compounds. It reacts rapidly with peroxynitrite (ONOO(-)) to form stable hydroxy derivatives. It undergoes Suzuki coupling with 4-bromotriphenylamine catalyzed by dichlorobis(triphenylphosphine)Pd(II), during the synthesis of dendrimers.
애플리케이션
4-Acetylphenylboronic acid was used in the synthesis of 4′-azidoacetophenone.
Reactant involved in:
- Palladium-catalyzed decarboxylative coupling
- Copper-catalyzed hydroxylation
- Palladium-catalyzed Suzuki-Miyaura cross-coupling
- Cross-coupling with α-bromocarbonyl compounds
- Oxidation catalyzed by Baeyer-Villiger monooxygenases
- 1,5-substitution reactions
기타 정보
Contains varying amounts of anhydride
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Adam Sikora et al.
Free radical biology & medicine, 47(10), 1401-1407 (2009-08-19)
In this study, we show that boronates, a class of synthetic organic compounds, react rapidly and stoichiometrically with peroxynitrite (ONOO(-)) to form stable hydroxy derivatives as major products. Using a stopped-flow kinetic technique, we measured the second-order rate constants for
Kimberly D Grimes et al.
Synthesis, 2010(9), 1441-1448 (2010-06-08)
We report the copper(II)-catalyzed conversion of organoboron compounds into the corresponding azide derivatives. A systematic series of phenylboronic acid derivatives is evaluated to examine the importance of steric and electronic effects of the substituents on reaction yield as well as
A New Efficient Convergent Synthesis of Conjugated Aryl-containing Dendrimers.
El-Deeb IM and Lee SH.
Bull. Korean Chem. Soc., 31(6), 1757-1760 (2010)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)


![1-[4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]ethanone AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/280/787/64aa2a50-1d44-4c16-ace9-c54ea40606e6/640/64aa2a50-1d44-4c16-ace9-c54ea40606e6.png)