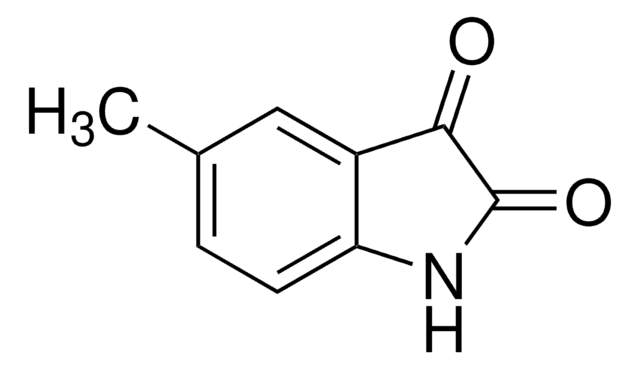
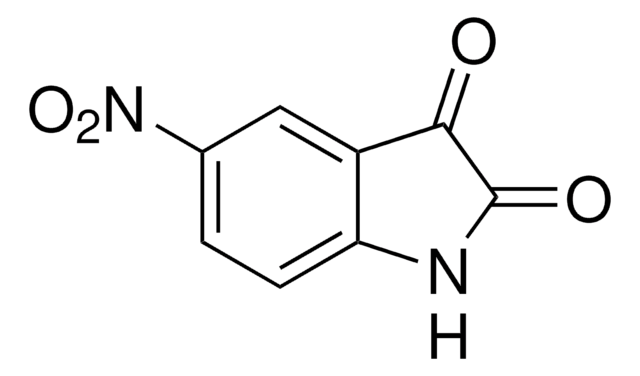
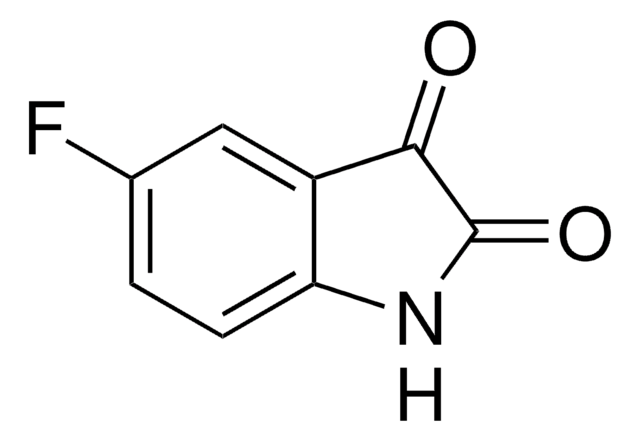
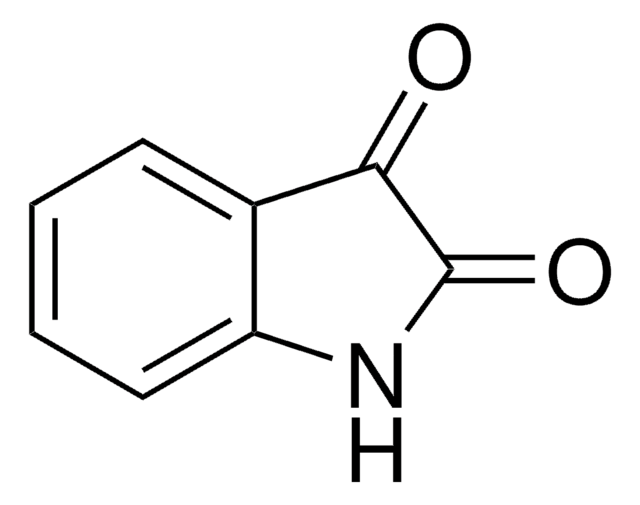
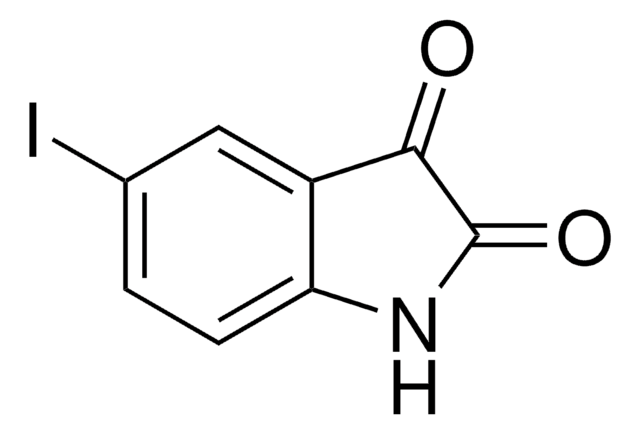
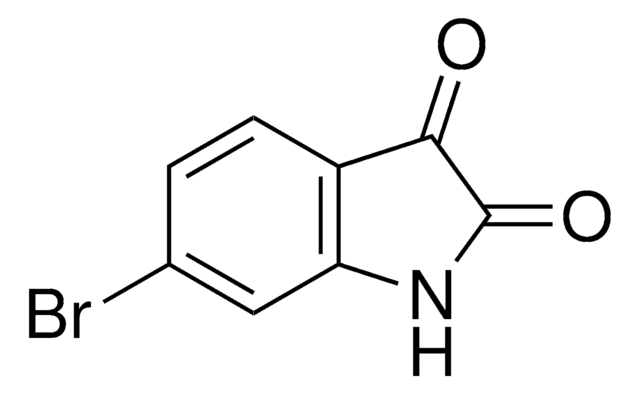
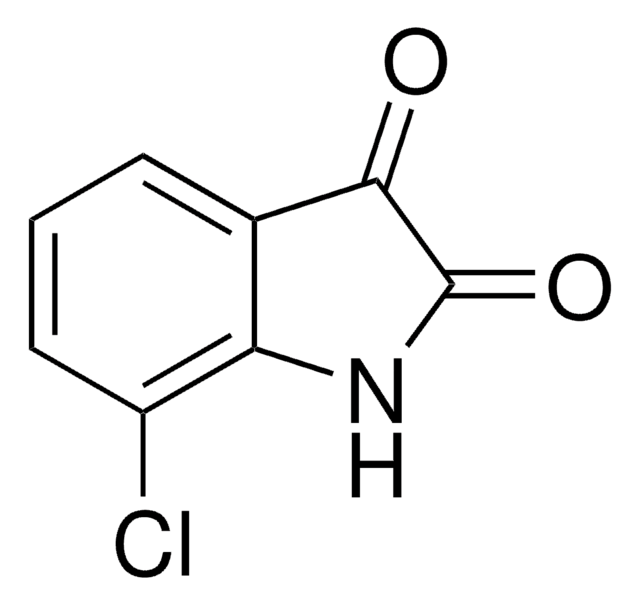
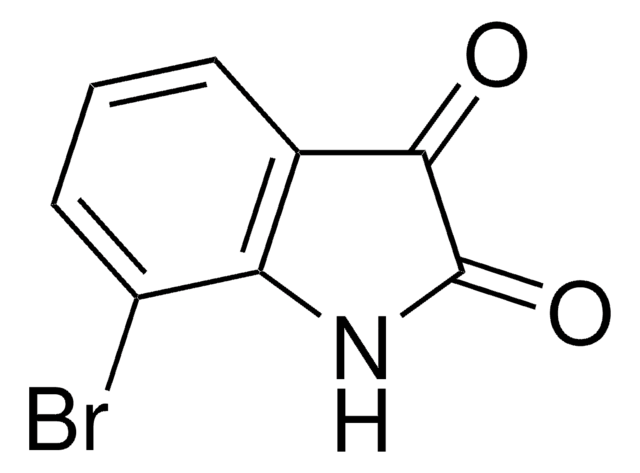
추천 제품
일반 설명
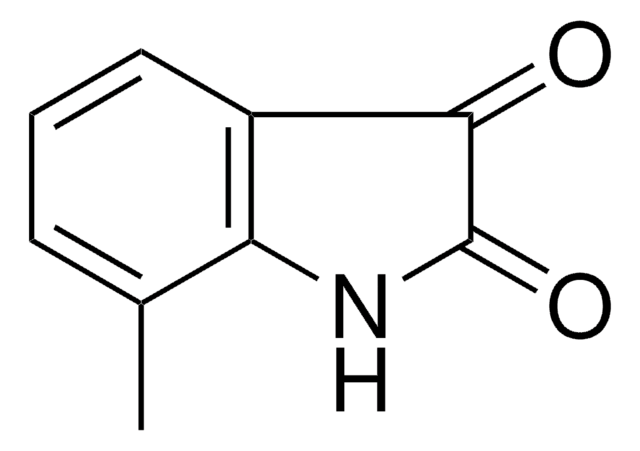
5-Bromoisatin is a 5-haloisatin. One of the methods reported for its synthesis is by reacting N-halosaccharins with isatin in the presence of SiO2. Its inotropic activity has been studied on rhythmically stimulated papillary muscles of guinea pigs. It is reported to exhibit analgesic and sedative properties at a dose of 0.2g/kg in mice.
애플리케이션
5-Bromoisatin may be used in the synthesis of the following:
- N-derivatives of 5-bromoisatin
- N-substituted pyrroles
- linear polyaryleneoxindoles
- 5-bromodioxindole
- cinchoninic acid derivatives
- 3-hydroxyoxindole
- S-benzyldithiocarbazate Schiff Bases
- 5-bromooxindole
- Morita-Baylis-Hillman adducts of isatin derivatives
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Synthesis and spectral data for cinchoninic acids.
Sarkis GY.
Journal of Chemical and Engineering Data, 17(3), 388-391 (1972)
Superelectrophiles in aromatic polymer chemistry.
Colquhoun HM, et al.
Macromolecules, 34(4), 1122-1124 (2001)
Electro-organic synthesis of nanosized particles of 3-hydroxy-3-(1H-indol-3-yl) indolin-2-one derivatives.
Makarem S, et al.
Monatshefte fur Chemie / Chemical Monthly, 148(8), 1157-1160 (2012)
Quick and efficient synthesis of Morita-Baylis-Hillman adducts of isatin derivatives.
Rad-Moghadam K and Youseftabar-Miri L.
ARKIVOC (Gainesville, FL, United States), 11, 43-50 (2011)
SiO2 mediated reaction of isatin with N-halosaccharins: A regiospecific preparation of 5-haloisatins.
de Souza SPL, et al.
Heterocyclic Communications, 9(1), 31-34 (2003)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.