477222
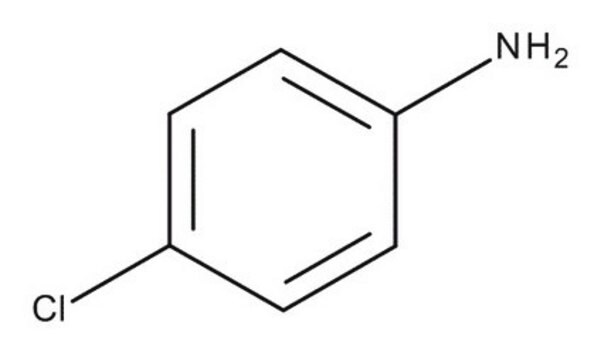
4-Chloroaniline
purified by sublimation, ≥99%
동의어(들):
1-Amino-4-chlorobenzene, 4-Chlorophenylamine, p-Chloroaniline
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
Linear Formula:
ClC6H4NH2
CAS Number:
Molecular Weight:
127.57
Beilstein:
471359
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
일반 설명
4-Chloroaniline (4-ClA) is a chlorinated aromatic amine that is formed as an intermediate during the microbial decomposition of phenylurea and phenylcarbamate. The formation of various oligomers by polymerization of 4-ClA with guaiacol in an aqueous solution containing oxidoreductases has been reported. 4-ClA undergoes decomposition via oxidation by fusarium oxysporum, a soil fungus.
애플리케이션
4-Chloroaniline may be used to synthesize:
- 4-chloronitrosobenzene
- 2-bromo-4-chloroaniline
- 2,6-dibromo-4-chloroaniline
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B - Skin Sens. 1
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
248.0 °F - closed cup
Flash Point (°C)
120.0 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
Oxidative co-oligomerization of guaiacol and 4-chloroaniline.
Simmons KE, et al.
Environmental Science & Technology, 23(1), 115-121 (1989)
The action of chloride peroxidase on 4-chloroaniline. N-oxidation and ring halogenation.
Corbett MD, et al.
The Biochemical Journal, 187(3), 893-903 (1980)
M D Corbett et al.
The Biochemical journal, 175(2), 353-360 (1978-11-01)
The incubation of 4-chloroaniline with chloroperoxidase and H2O2 resulted in a rapid formation of 4-chloronitrosobenzene. This enzymic oxidation displayed a pH optimum at 4.4 with a Km of 8.1x10(-4)M and catalytic-centre activity of 312. The initial rate of the reaction
Microbial oxidation of 4-chloroaniline.
D D Kaufman et al.
Journal of agricultural and food chemistry, 21(1), 127-132 (1973-01-01)
Shashikala Krishnamurthy et al.
Journal of endodontics, 36(7), 1154-1157 (2010-07-16)
The purpose of this study was (1) to evaluate maximum thickness the and chemical composition of the precipitate formed between sodium hypochlorite (NaOCl) and chlorhexidine (CHX) and (2) to evaluate effectiveness of absolute alcohol to remove residual NaOCl and thereby
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.