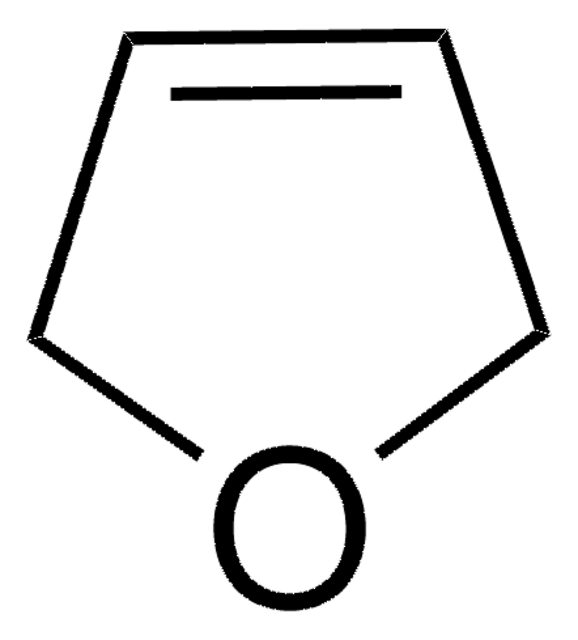
추천 제품
Quality Level
분석
96%
refractive index
n20/D 1.458 (lit.)
bp
208 °C (lit.)
density
0.981 g/mL at 25 °C (lit.)
SMILES string
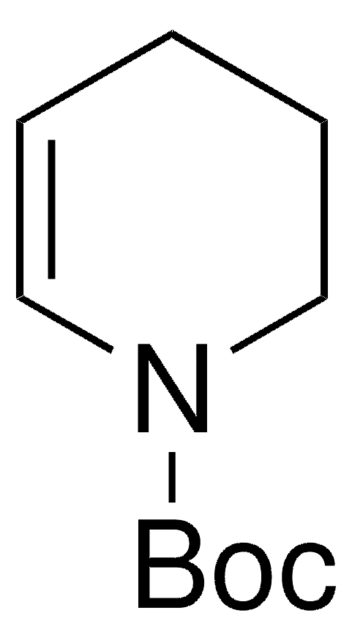
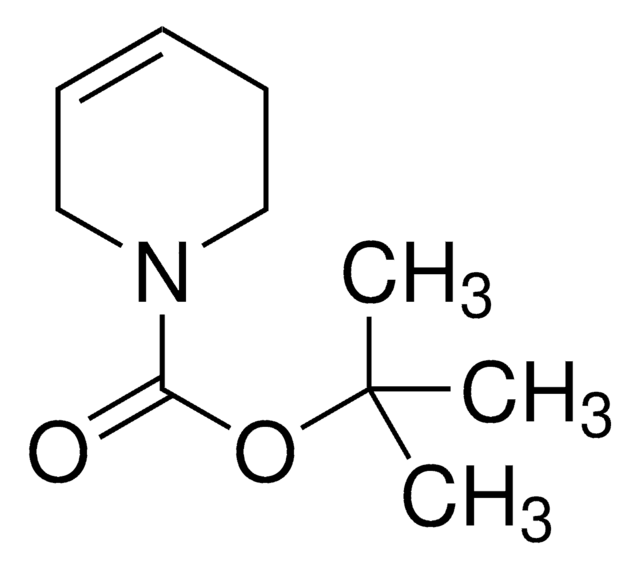
CC(C)(C)OC(=O)N1CC=CC1
InChI
1S/C9H15NO2/c1-9(2,3)12-8(11)10-6-4-5-7-10/h4-5H,6-7H2,1-3H3
InChI key
YEBDZDMYLQHGGZ-UHFFFAOYSA-N
일반 설명
N-Boc-2,5-dihydro-1H-pyrrole, also known as tert-butyl 2,5-dihydro-1H-pyrrole-1-carboxylate, can be synthesized from N-boc-diallylamine.
애플리케이션
N-Boc-2,5-dihydro-1H-pyrrole (tert-Butyl 2,5-dihydro-1H-pyrrole-1-carboxylate) may be used in the preparation of tert-butyl 3-aryl-2,3-dihydro-1H-pyrrole-1-carboxylate.
Used in a synthesis of ß-aryl-GABA analogues by Heck arylation with arenediazonium salts.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
178.0 °F
Flash Point (°C)
81.1 °C
개인 보호 장비
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
가장 최신 버전 중 하나를 선택하세요:
A Short and Efficient Synthesis of (S)-1-Boc-2, 5-dihydro-1H-pyrrole-2-carboxylic Acid.
Sturmer R, et al.
Synthesis, 1, 46-48 (2001)
Synthesis of aryl pyrrolizidines from endocyclic enecarbamates. Novel applications of the Heck arylation of 3-pyrrolines using diazonium salts.
de Oca ACBM and Correia CRD.
ARKIVOC (Gainesville, FL, United States), 10, 390-403 (2003)
Ariel L L Garcia et al.
The Journal of organic chemistry, 70(3), 1050-1053 (2005-01-29)
We report herein a new, practical, and economic synthesis of the phosphodiesterase inhibitor Rolipram on a multigram scale as well as the synthesis of new 4-aryl pyrrolidones and beta-aryl-gamma-amino butyric acids (GABA derivatives) employing an efficient Heck-Matsuda arylation of 3-pyrroline
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
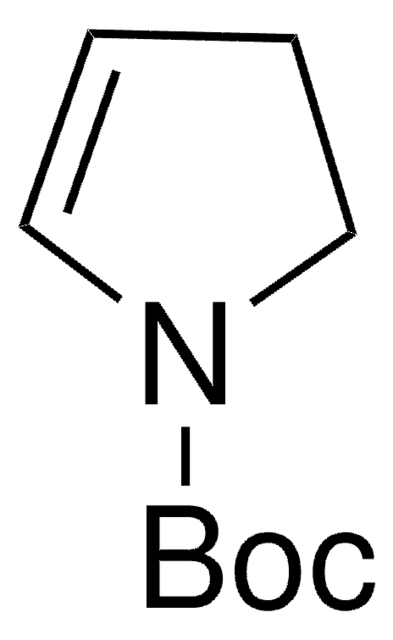
![(1S,4S)-(−)-2-Boc-2,5-diazabicyclo[2.2.1]heptane 95%](/deepweb/assets/sigmaaldrich/product/structures/401/063/1eb3be94-6385-4815-8b42-ff76c097cc27/640/1eb3be94-6385-4815-8b42-ff76c097cc27.png)