479500
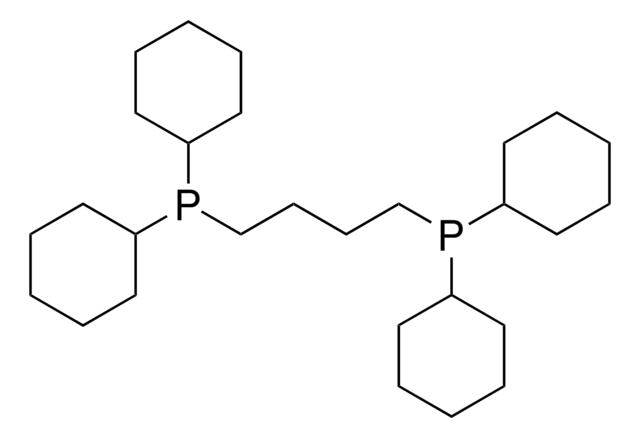
1,2-Bis(dicyclohexylphosphino)ethane
동의어(들):
Ethylenebis(dicyclohexylphosphine), 1,2-Ethanediylbis[dicyclohexyl]phosphine
로그인조직 및 계약 가격 보기
모든 사진(3)
About This Item
Linear Formula:
(C6H11)2PCH2CH2P(C6H11)2
CAS Number:
Molecular Weight:
422.61
MDL number:
UNSPSC 코드:
12352001
PubChem Substance ID:
NACRES:
NA.22
추천 제품
양식
solid
Quality Level
반응 적합성
reagent type: ligand
reaction type: Decarboxylations
reagent type: ligand
reaction type: Negishi Coupling
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling
mp
92.5-96.5 °C (lit.)
작용기
phosphine
SMILES string
C1CCC(CC1)P(CCP(C2CCCCC2)C3CCCCC3)C4CCCCC4
InChI
1S/C26H48P2/c1-5-13-23(14-6-1)27(24-15-7-2-8-16-24)21-22-28(25-17-9-3-10-18-25)26-19-11-4-12-20-26/h23-26H,1-22H2
InChI key
BOUYBUIVMHNXQB-UHFFFAOYSA-N
애플리케이션
1,2-Bis(dicyclohexylphosphino)ethane can be used as a ligand for:
- Pd-catalyzed decarbonylative C-H coupling of azoles and aromatic esters.
- Ni-catalyzed cross-coupling reaction of aryl fluorides and primary amines.
Reactant involved in:
Precursor for Iridium trisboryl complexes and the substituent effect on borylation reactions
Ligand for palladium(II) complex catalyzed hydrogenation reactions
- Investigations of the role of ligand-based steric effects during the polymerization
- Synthesis of molybdenum nitrosyl complexes for use as Imine hydrogenation catalysts
- Irreversible thermal linkage isomerization of switchable C-N-bound isomers
- Chelating for conversion of trans complexes to cis complexes
Precursor for Iridium trisboryl complexes and the substituent effect on borylation reactions
Ligand for palladium(II) complex catalyzed hydrogenation reactions
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Aquatic Chronic 4 - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
>230.0 °F - closed cup
Flash Point (°C)
> 110 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Pd-Catalyzed Decarbonylative C- H Coupling of Azoles and Aromatic Esters.
Matsushita K, et al.
Chemistry - An Asian Journal (2018)
Nickel-catalyzed amination of aryl fluorides with primary amines.
Harada T, et al.
Chemical Communications (Cambridge, England), 54(14), 1718-1721 (2018)
Helen Larson et al.
The Journal of organic chemistry, 84(20), 13092-13103 (2019-09-25)
This manuscript details the development of the nickel-catalyzed arylation of oxazoles and benzoxazoles with aryl halides. A series of aryl, heteroaryl, and druglike electrophiles relevant to pharmaceutical applications were surveyed. The desired arylated products were obtained in synthetically useful yields
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.







![[1,1′-Bis(di-cyclohexylphosphino)ferrocene]dichloropalladium(II) 98%](/deepweb/assets/sigmaaldrich/product/structures/136/854/a3142b2e-900c-47e5-8100-e48add9f4db6/640/a3142b2e-900c-47e5-8100-e48add9f4db6.png)