모든 사진(1)
About This Item
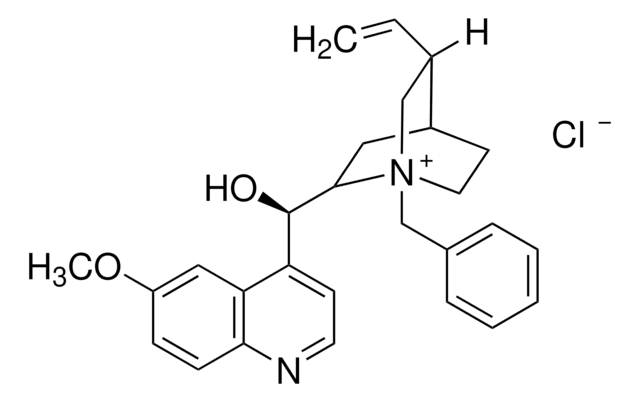
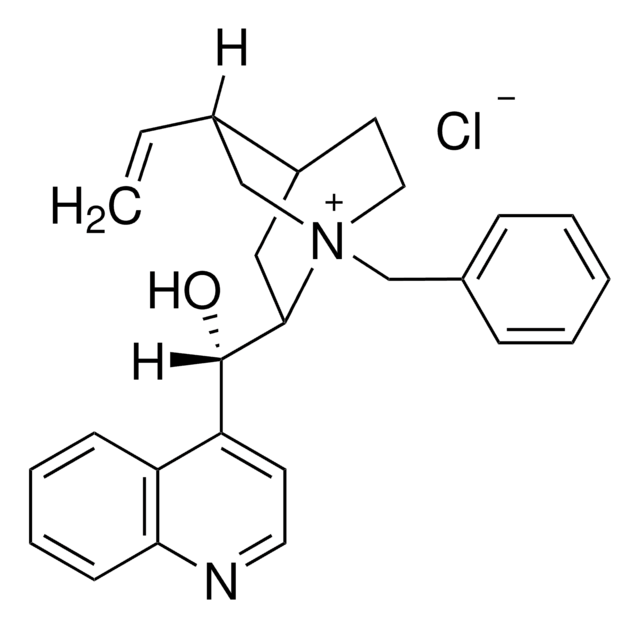
실험식(Hill 표기법):
C26H29BrN2O
CAS Number:
Molecular Weight:
465.43
MDL number:
UNSPSC 코드:
12352101
NACRES:
NA.22
추천 제품
Quality Level
분석
97%
광학 활성
[α]20/D −138°, c = 1 in chloroform
mp
190 °C (dec.) (lit.)
InChI key
UUVFTQCFSNVLGV-SPTWEYDNSA-M
일반 설명
N-Benzylcinchonidinium bromide is a chiral phase-transfer catalyst (PTC).
애플리케이션
N-Benzylcinchonidinium bromide (BCDBr) can be used:
- To prepare various 4-(bromomethyl)benzenesulfonamides, which are employed as catalysts for asymmetric benzylation reactions.
- As a phase transfer catalyst in the synthesis of organogelators via Michael addition reaction.
- To catalyze the enantioselective alkylation of tert-butyl glycinate-benzophenone Schiff base furnishing the corresponding chiral α-amino acid.
- To resolve symmetric biaryldiols via formation of inclusion complex with diols.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
Separation of the Enantiomers of 2, 2?-Dihydroxy-1, 1?-binaphthyl and 10, 10?-Dihydroxy-9, 9?-biphenanthryl by Complexation with N-Alkylcinchonidinium Halides
Tanaka K, et al.
Angewandte Chemie (International ed. in English), 32(8), 1147-1148 (1993)
Enantioselective reactions of tert-butyl glycinate-benzophenone Schiff base catalyzed by chiral phase-transfer catalyst in aqueous media without any organic solvent.
Mase N, et al.
Tetrahedron Letters, 46(18), 3213-3216 (2005)
Synthesis of green organogelators with a sulfide linkage via solvent-free Michael addition: soft templates for the preparation of size-controlled gold nanoparticles
Delbecq F, et al.
Tetrahedron Letters, 54(7), 651-656 (2013)
Synthesis of cinchonidinium salts containing sulfonamide functionalities and their catalytic activity in asymmetric alkylation reactions
Itsuno S, et al.
Tetrahedron Letters, 55(44), 6117-6120 (2014)
문서
Maruoka catalysts enable enantioselective preparation of α-amino acids from glycine derivatives via asymmetric phase transfer catalysis.
Asymmetric phase-transfer catalysis (PTC) has been recognized as a “green” alternative to many homogeneous synthetic organic transformations, and has found widespread application. Synthetically modified cinchona alkaloids are typical chiral organocatalysts used in asymmetric PTC.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.![(11bS)-(+)-4,4-Dibutyl-4,5-dihydro-2,6-bis(3,4,5-trifluorophenyl)-3H-dinaphth[2,1-c:1′,2′-e]azepinium bromide](/deepweb/assets/sigmaaldrich/product/structures/230/279/5c1a7e7e-f791-4612-9920-56c6d7c4f735/640/5c1a7e7e-f791-4612-9920-56c6d7c4f735.png)

![(11bR)-(–)-4,4-Dibutyl-4,5-dihydro-2,6-bis(3,4,5-trifluorophenyl)-3H-dinaphth[2,1-c:1′,2′-e]azepinium bromide Nagase purity](/deepweb/assets/sigmaaldrich/product/structures/147/005/c2a47bf2-5270-4469-9f3e-138f76f8f4c3/640/c2a47bf2-5270-4469-9f3e-138f76f8f4c3.png)