525057
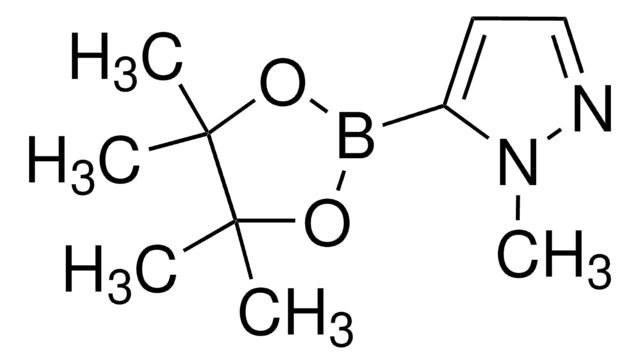
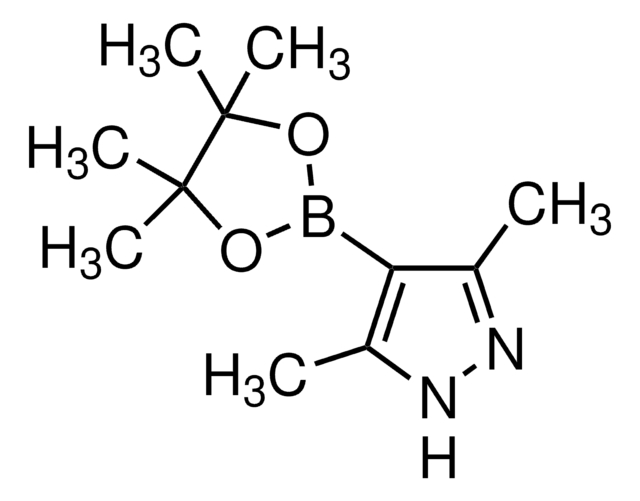
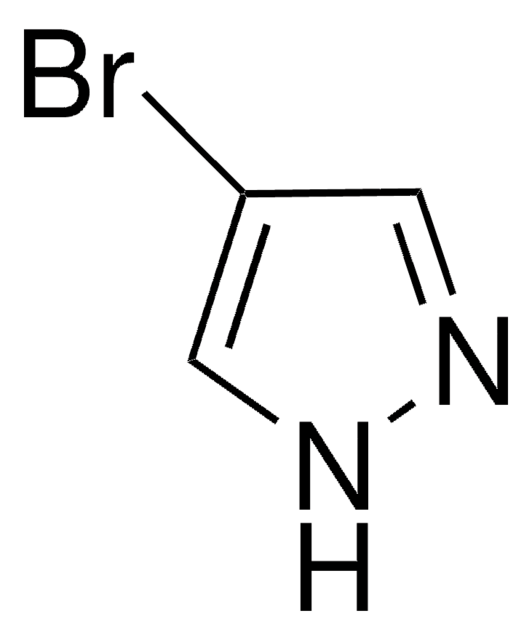
4-Pyrazoleboronic acid pinacol ester
97%
동의어(들):
4,4,5,5-Tetramethyl-2-(1H-pyrazol-2-yl)-1,3,2-dioxaborolane, 4,4,5,5-Tetramethyl-2-(1H-pyrazol-4-yl)-1,3,2-dioxaborolane, 4,4,5,5-Tetramethyl-2-(pyrazol-4-yl)-1,3,2-dioxaborolane, 4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)pyrazole, Pyrazol-4-ylboronic acid pinacol ester
로그인조직 및 계약 가격 보기
모든 사진(3)
About This Item
Linear Formula:
(CH3)4C2O2BC3N2H3
CAS Number:
Molecular Weight:
194.04
MDL number:
UNSPSC 코드:
12352103
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
97%
양식
solid
mp
142-146 °C (lit.)
SMILES string
CC1(C)OB(OC1(C)C)c2cn[nH]c2
InChI
1S/C9H15BN2O2/c1-8(2)9(3,4)14-10(13-8)7-5-11-12-6-7/h5-6H,1-4H3,(H,11,12)
InChI key
TVOJIBGZFYMWDT-UHFFFAOYSA-N
애플리케이션
Reagent used for
Reagent used in preparation of inhibitors of many highly significant therapeutic enzymes and kinases containing the privileged scaffold pyrazole, including
- Suzuki-Miyaura cross-couplings
- Ruthenium-catalyzed asymmetric hydrogenation
Reagent used in preparation of inhibitors of many highly significant therapeutic enzymes and kinases containing the privileged scaffold pyrazole, including
- VEGF
- Aurora
- Rho (ROCK)
- Janus Kinase 2 (JAK)
- c-MET
- ALK
- S-nitrosoglutathione reductase
- CDC7
- Acetyl-CoA carboxylase
- Prosurvival Bcl-2 protein
- Viral RNA-Dependent RNA polymerase
- Long Chain Fatty Acid Elongase 6
- PI3
- AKT
- Chk1
- Protein Kinase B
법적 정보
Product of Boron Molecular
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Asymmetric synthesis of potent chroman-based Rho kinase (ROCK-II) inhibitors
Chen, Y-T.; et al.
MedChemComm, 2, 73-75 (2011)
Hassan M Shallal et al.
Bioorganic & medicinal chemistry letters, 21(5), 1325-1328 (2011-02-09)
Overexpression of prosurvival or underexpression of pro-death Bcl-2 family proteins can lead to cancer cell resistance to chemotherapy and radiation treatment. Inhibition of the prosurvival Bcl-2 family proteins has become a strategy for cancer therapy and inhibitors are currently being
Michael L Curtin et al.
Bioorganic & medicinal chemistry letters, 22(9), 3208-3212 (2012-04-03)
In an effort to identify multi-targeted kinase inhibitors with a novel spectrum of kinase activity, a screen of Abbott proprietary KDR inhibitors against a broad panel of kinases was conducted and revealed a series of thienopyridine ureas with promising activity
Robert M Garbaccio et al.
Bioorganic & medicinal chemistry letters, 17(22), 6280-6285 (2007-09-29)
From HTS lead 1, a novel benzoisoquinolinone class of ATP-competitive Chk1 inhibitors was devised and synthesized via a photochemical route. Using X-ray crystallography as a guide, potency was rapidly enhanced through the installation of a tethered basic amine designed to
Mika Lindvall et al.
ACS medicinal chemistry letters, 2(10), 720-723 (2011-10-13)
A ligand-based 3D pharmacophore model for serine/threonine kinase CDC7 inhibition was created and successfully applied in the discovery of novel 2-(heteroaryl)-6,7-dihydrothieno[3,2-c]pyridin-4(5H)-ones. The pharmacophore model provided a hypothesis for lead generation missed by docking to a homology model. Medicinal chemistry exploration
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)