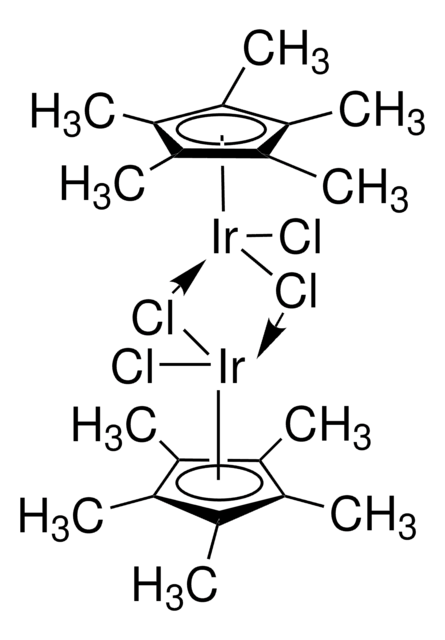
추천 제품
양식
crystals
Quality Level
반응 적합성
core: iridium
reagent type: catalyst
reaction type: C-H Activation
mp
154-179 °C (D)
저장 온도
−20°C
SMILES string
C[O+]1[Ir-]2[O+](C)[Ir-]12.C3CC=CCCC=C3.C4CC=CCCC=C4
InChI
1S/2C8H12.2CH3O.2Ir/c2*1-2-4-6-8-7-5-3-1;2*1-2;;/h2*1-2,7-8H,3-6H2;2*1H3;;/q;;2*+1;2*-1/b2*2-1-,8-7-;;;;
InChI key
BGWIAAATAAWGOI-MIXQCLKLSA-N
애플리케이션
A powerful C-H activation catalyst to prepare phenols from arenes
Catalyst for:
- Preparation of heteroaryl fused indole ring systems as inhibitors of HCV NS5B polymerase
- Borylation/Suzuki-Miyaura coupling
- Metalation-Suzuki cross-coupling procedure for the synthesis of biaryls and heterobiaryls
- Tetraborylation reactions
- Highly regio- and enantioselective asymmetric hydroboration
- Ortho-silylation of aryl ketone, benzaldehyde, and benzyl alcohol derivatives via C-H activation
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
A general strategy for the perfluoroalkylation of arenes and arylbromides by using arylboronate esters and [(phen) CuRF].
Litvinas N, et al.
Angewandte Chemie (International Edition in English), 51(2), 536-539 (2012)
Ir?Catalyzed Borylation of C H Bonds in N?Containing Heterocycles: Regioselectivity in the Synthesis of Heteroaryl Boronate Esters.
Mkhalid I, et al.
Angewandte Chemie (International Edition in English), 45(3), 489-491 (2006)
Catalytic functionalization of unactivated primary C?H bonds directed by an alcohol.
Simmons E, et al.
Nature, 483(7387), 70-70 (2012)
Direct C?H borylation and C?H arylation of pyrrolo [2, 3-d] pyrimidines: synthesis of 6, 8-disubstituted 7-deazapurines.
Klecka M, et al.
Organic & Biomolecular Chemistry, 7(5), 866-868 (2009)
Robert E Maleczka et al.
Journal of the American Chemical Society, 125(26), 7792-7793 (2003-06-26)
An efficient one-pot C-H activation/borylation/oxidation protocol for the preparation of phenols is described. This method is particularly attractive for the generation of meta-substituted phenols bearing ortho-/para-directing groups, as such substrates are difficult to access by other phenol syntheses.
문서
Arylboronic acids and esters, vital tools in chemical transformations, find extensive use, particularly in the Suzuki-Miyaura cross-coupling reaction.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

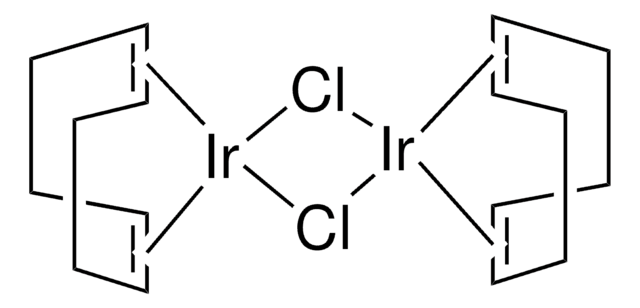




![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)