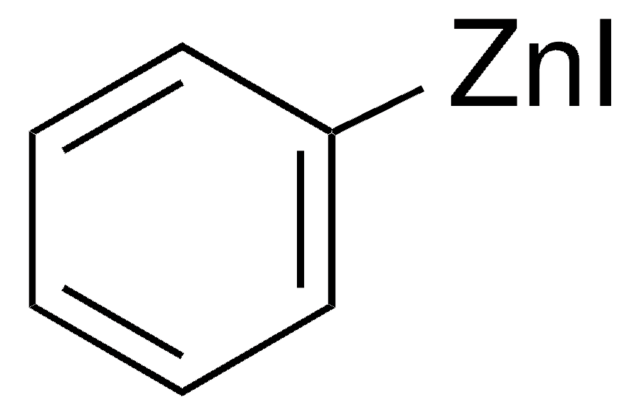
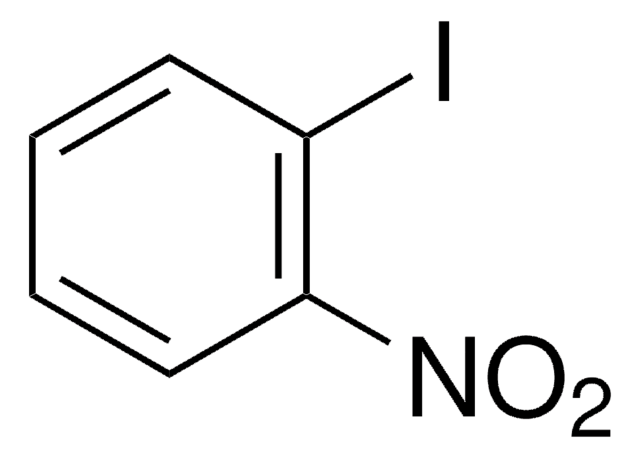
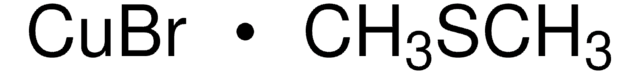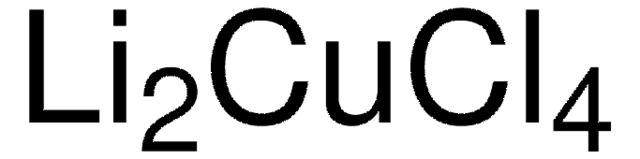추천 제품
형태
liquid
Quality Level
반응 적합성
core: copper
reagent type: catalyst
농도
in anhydrous tetrahydrofuran
density
0.999 g/mL at 25 °C
SMILES string
[Li]Cl.[Li]Cl.[Cu]C#N
InChI
1S/CN.2ClH.Cu.2Li/c1-2;;;;;/h;2*1H;;;/q;;;;2*+1/p-2
InChI key
QGXKBLXNBYNHBV-UHFFFAOYSA-L
애플리케이션
Copper(I) cyanide di(lithium chloride) complex can be used for the synthesis of organocopper(I) reagents by transmetalation with organozinc and Grignard reagents. It is also a useful precursor to prepare organocuprate(I) reagents.
신호어
Danger
Hazard Classifications
Acute Tox. 1 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 2 - Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Central nervous system, Respiratory system
보충제 위험성
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point (°F)
1.4 °F - closed cup - Solvent
Flash Point (°C)
-17 °C - closed cup - Solvent
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Regioselective functionalization of trisubstituted pyridines using a bromine-magnesium exchange.
Ren H and Knochel P
Chemical Communications (Cambridge, England), 11(7), 726-728 (2006)
Krause, N.; Gerold, A.
Angewandte Chemie (International Edition in English), 36, 186-186 (1997)
TMPZnCl?LiCl: A new active selective base for the directed zincation of sensitive aromatics and heteroaromatics.
Mosrin M and Knochel P
Organic Letters, 11(8), 1837-1840 (2009)
Joel M Harris et al.
The Journal of organic chemistry, 68(11), 4371-4381 (2003-05-24)
2,5,6-Trisubstituted piperidines are readily prepared by a combination of an aza-Achmatowicz oxidation of a furyl-substituted benzenesulfonamide followed by a conjugate addition to the resulting 2H-pyridone and subsequent addition of various nucleophiles to a transient N-sulfonyliminium ion. The stereochemistry of the
Tomas Hudlicky et al.
The Journal of organic chemistry, 67(25), 8726-8743 (2002-12-07)
Biocatalytic approaches have yielded efficient total syntheses of the major Amaryllidaceae alkaloids, all based on the key enzymatic dioxygenation of suitable aromatic precursors. This paper discusses the logic of general synthetic design for lycoricidine, narciclasine, pancratistatin, and 7-deoxypancratistatin. Experimental details
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.