추천 제품
형태
powder
Quality Level
반응 적합성
reagent type: catalyst
reaction type: Photocatalysis
mp
346-351 °C
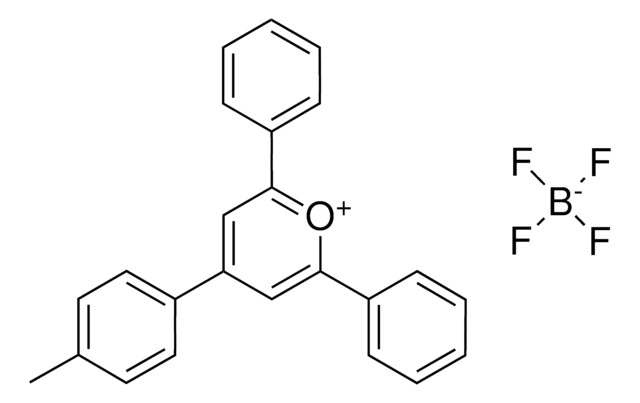
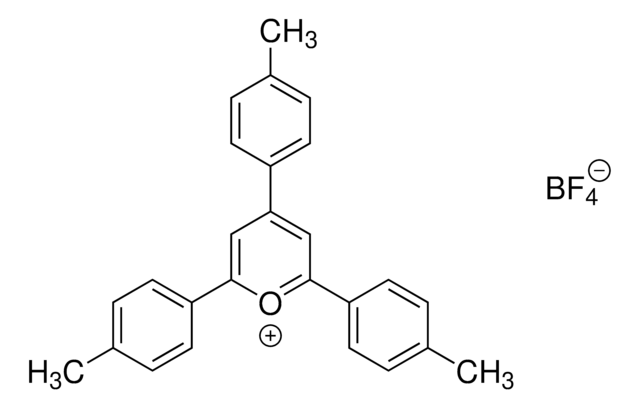
SMILES string
COC(C=C1)=CC=C1C2=[O+]C(C3=CC=C(OC)C=C3)=CC(C4=CC=C(OC)C=C4)=C2.FB(F)F.[F-]
애플리케이션
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
기타 정보
Cyclization–endoperoxidation cascade reactions of dienes mediated by a pyrylium photoredox catalyst
Metal-Free Ring-Opening Metathesis Polymerization
Cationic Polymerization of Vinyl Ethers Controlled by Visible Light
Electron-Transfer-Induced Diels ± Alder Reactions of Indole and Exocyclic Dienes: Synthesis and Quantum-Chemical Studies
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
관련 콘텐츠
The Nicewicz lab is focused on the discovery of new and powerful reaction methodologies that proceed via the intermediacy of highly reactive cation radical species. Included in these transformations are anti-Markovnikov selective additions of amines, alcohols, carboxylic acids, amides and mineral acids to alkenes.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
![[Ir(dFCF3ppy)2-(5,5’-dCF3bpy)]PF6 ≥95%](/deepweb/assets/sigmaaldrich/product/structures/422/901/e00f3148-fb86-4f94-9e79-21d064c3f327/640/e00f3148-fb86-4f94-9e79-21d064c3f327.png)