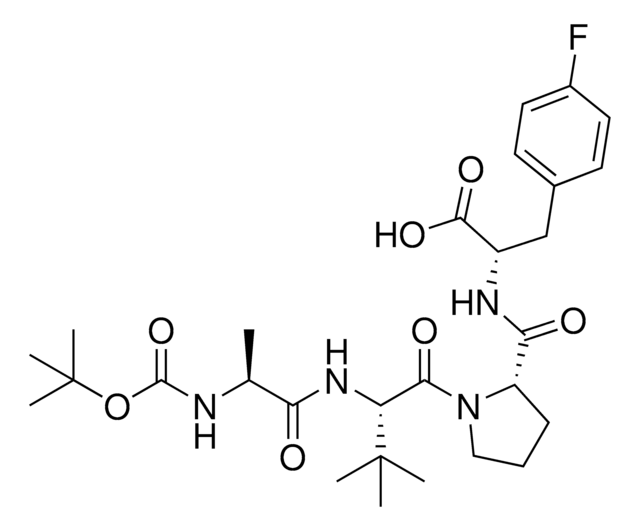
916714
A1V2PF2-NHEt
≥95%
동의어(들):
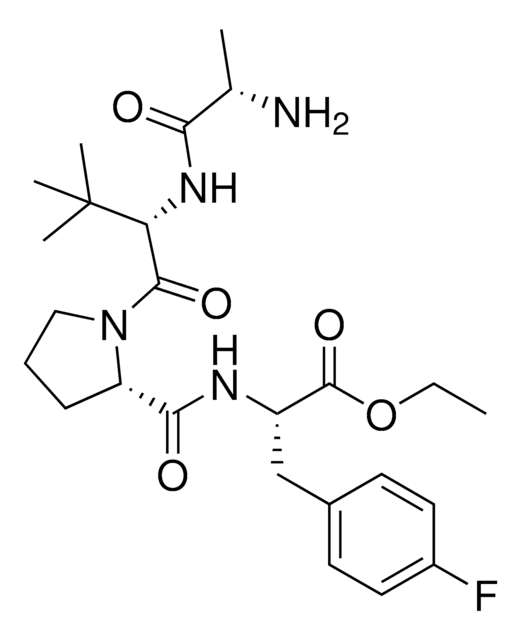
(S)-1-((S)-2-((S)-2-Aminopropanamido)-2-cyclohexylacetyl)-N-((S)-1-(ethylamino)-3-(4-fluorophenyl)-1-oxopropan-2-yl)pyrrolidine-2-carboxamide, AVP ligand, IAP E3 ligase lead for protein degrader research, SNIPER building block
About This Item
추천 제품
ligand
A1V2PF2
Quality Level
분석
≥95%
형태
powder
반응 적합성
reagent type: ligand
작용기
amine
저장 온도
2-8°C
SMILES string
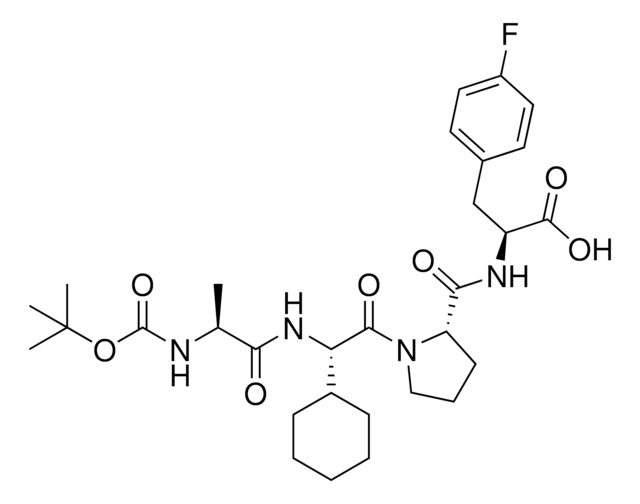
N[C@H](C(N[C@H](C(N1CCC[C@H]1C(N[C@H](C(NCC)=O)CC2=CC=C(C=C2)F)=O)=O)C3CCCCC3)=O)C
애플리케이션
A1V2PF2-NHEt conjugates are also available for degrader synthesis. Browse our full synthesis offering here for streamlining SNIPER and PROTAC® degrader libraries: Degrader Building Blocks with Inhibitor of Apoptosis Protein (IAP) In Silico-Derived Ligands
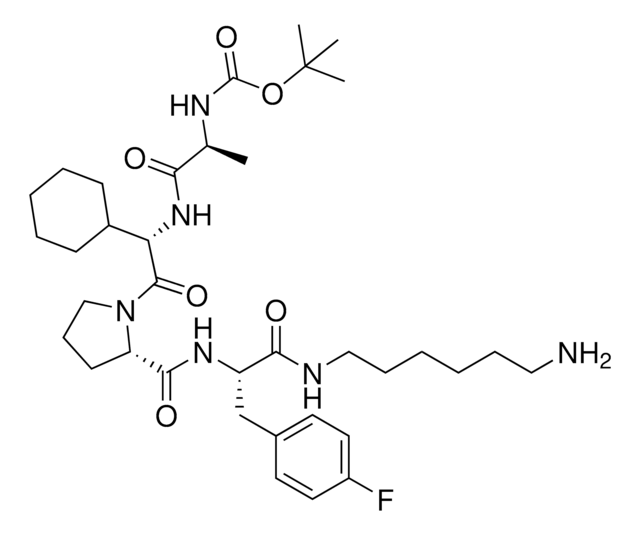
917931 A1V2PF2-NHEt-C6-NH2
916684 A1V2PF2-NHEt-C10-NH2
916935 A1V2PF2-NHEt-PEG1-NH2
917192 A1V2PF2-NHEt-PEG3-NH2
기타 정보
In Vivo Knockdown of Pathogenic Proteins via Specific and Nongenetic Inhibitor of Apoptosis Protein (IAP)-dependent Protein Erasers (SNIPERs)
SNIPERs−Hijacking IAP activity to induce protein degradation
E3 Ligase Ligands for PROTACs: How They Were Found and How to Discover New Ones
법적 정보
관련 제품
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
문서
Targeted protein degradation reduces disease-relevant proteins in cells using small molecules, hijacking endogenous proteolysis systems.
Plate of 80 ligands against E3 ligase IAP designed by ComInnex; allows creation of bifunctional targeted protein degraders or molecular glues.
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.