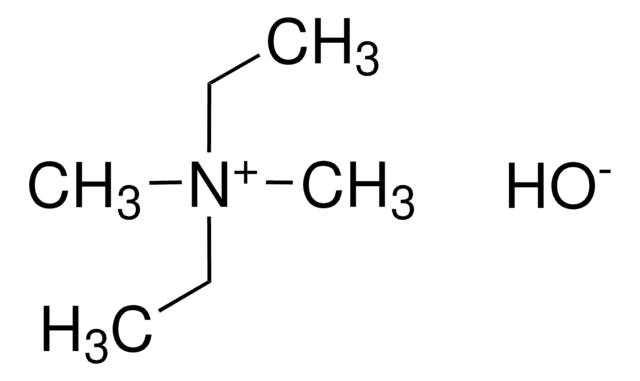
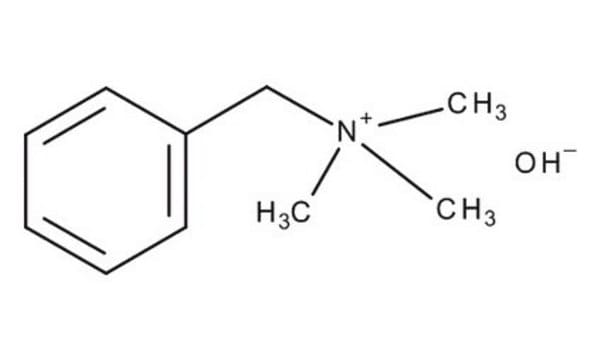
B32602
Benzyltrimethylammonium hydroxide solution
40 wt. % in methanol
동의어(들):
N,N,N-Trimethyl-N-benzylammonium hydroxide, N,N,N-trimethyl-1-phenylmethanaminium hydroxide, N,N,N-trimethylbenzenemethanaminium hydroxide, Triton B
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
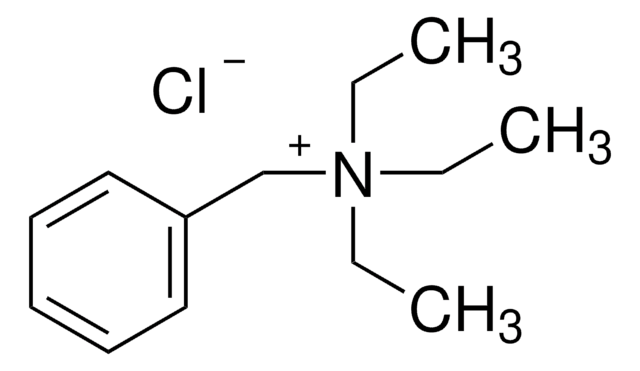
C6H5CH2N(OH)(CH3)3
CAS Number:
Molecular Weight:
167.25
Beilstein:
3917256
MDL number:
UNSPSC 코드:
12352005
PubChem Substance ID:
NACRES:
NA.22
추천 제품
양식
liquid
농도
40 wt. % in methanol
density
0.92 g/mL at 25 °C
SMILES string
[OH-].C[N+](C)(C)Cc1ccccc1
InChI
1S/C10H16N.H2O/c1-11(2,3)9-10-7-5-4-6-8-10;/h4-8H,9H2,1-3H3;1H2/q+1;/p-1
InChI key
NDKBVBUGCNGSJJ-UHFFFAOYSA-M
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Benzyltrimethylammonium hydroxide solution is a quaternary ammonium compound that is commonly used as a base in organic synthesis. It is also used as a base catalyst in some polymerization reactions.
애플리케이션
Benzyltrimethylammonium hydroxide solution can be used as:
- A catalyst in the nitroaldol condensation reaction
- A structure-directing agent in the synthesis of high-silica aluminosilicate zeolite chabazite type zeolite by hydrothermal method
- A ionic liquid precursor for the fabrication of nanostructured ZnO particles
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1A - STOT SE 1
표적 기관
Eyes,Central nervous system
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point (°F)
51.8 °F - closed cup
Flash Point (°C)
11 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles
이미 열람한 고객
Weisheng Lin et al.
Analytica chimica acta, 583(1), 98-102 (2007-03-28)
The importance of benzyltriethyl ammonium chloride (BTEAC) in industrial applications has stimulated the development of a number of methods for its determination. In this paper, a high performance capillary electrophoresis (CE) method, coupled with an extraction technique for determining BTEAC
J M Sanders et al.
Xenobiotica; the fate of foreign compounds in biological systems, 25(3), 303-313 (1995-03-01)
1. Benzyltrimethylammonium chloride (BTMAC)-derived radioactivity was rapidly eliminated from the F344 rat and the B6C3F1 mouse following p.o. administration of 0.63-63 mg/kg of [ring-U-14C] BTMAC. Greater than 90% of the radioactivity was excreted in urine and faeces within 24-h post-dosing.
Purushothaman Gopinath et al.
The Journal of organic chemistry, 74(16), 6291-6294 (2009-07-22)
An efficient protocol is reported for the synthesis of thioesters from carboxylic acids with use of acyloxy phosphonium salts as intermediates and benzyltriethylammonium tetrathiomolybdate as the sulfur transfer reagent.
L Sangeetha Vedula et al.
Archives of biochemistry and biophysics, 466(2), 260-266 (2007-08-07)
Trichodiene synthase is a terpenoid cyclase that catalyzes the cyclization of farnesyl diphosphate (FPP) to form the bicyclic sesquiterpene hydrocarbon trichodiene (89%), at least five sesquiterpene side products (11%), and inorganic pyrophosphate (PP(i)). Incubation of trichodiene synthase with 2-fluorofarnesyl diphosphate
Lena Edström et al.
Journal of chromatography. A, 1218(15), 1966-1973 (2010-10-05)
It has recently been demonstrated, using mathematical models, how peculiar overloaded band profiles of basic compounds are due to the local pH in the column when using low capacity buffers. In this study, overloaded peak shapes resulting after injection of
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)



![1,4-Diazabicyclo[2.2.2]octane ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)