모든 사진(2)
About This Item
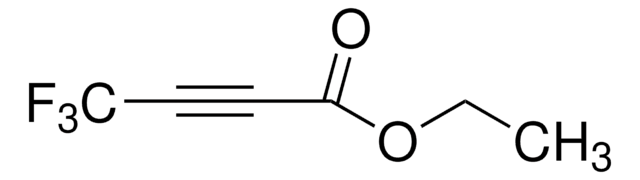
Linear Formula:
CH3OCOC≡CCO2CH3
CAS Number:
Molecular Weight:
142.11
Beilstein:
607063
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
일반 설명
Dimethyl acetylenedicarboxylate (DMAD) is an ester utilized as a dienophile and a dipolarophile in cycloaddition reactions.
애플리케이션
Versatile dienophile used in Diels-Alder reactions.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
186.8 °F - closed cup
Flash Point (°C)
86 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
Yuanyuan Chen et al.
Journal of the American Chemical Society, 129(35), 10773-10784 (2007-08-19)
Recently, it was reported that both dienylfurans and dienylisobenzofurans could react with dimethyl acetylenedicarboxylate (DMAD) to give [8+2] cycloadducts. Understanding these [8+2] reactions will aid the design of additional [8+2] reactions, which have the potential for the synthesis of 10-membered
Shunmin He et al.
Nature communications, 10(1), 2219-2219 (2019-05-19)
A long-standing question in the field of embryogenesis is how the zygotic genome is precisely activated by maternal factors, allowing normal early embryonic development. We have previously shown that N6-methyladenine (6mA) DNA modification is highly dynamic in early Drosophila embryos
Qiuping Ding et al.
The Journal of organic chemistry, 74(2), 921-924 (2008-12-06)
Tandem electrophilic cyclization-[3+2] cycloaddition-rearrangement reactions of 2-alkynylbenzaldoximes, DMAD, and bromine are described, which afford the unexpected isoquinoline-based azomethine ylides in good to excellent yields. The products could be further elaborated via palladium-catalyzed cross-coupling reactions to generate highly functionalized isoquinoline-based stable
Cheng Ma et al.
The Journal of organic chemistry, 70(22), 8919-8923 (2005-10-22)
[reaction: see text] A facile preparation of 3-aminofuran derivatives via multicomponent reactions of thiazole carbenes, aldehydes, and dimethyl acetylenedicarboxylate (DMAD) is reported. In this process, the thiazole carbenes, generated in situ from thiazolium salts, reacted with aldehydes and DMAD at
Yi Li et al.
Polymers, 13(3) (2021-01-28)
Big spherulite structure and high crystallinity are the two main drawbacks of poly(butylene succinate) (PBS) and hinder its application. In this work, a new type of copolyester poly(butylene succinate-co-butylene acetylenedicarboxylate) (PBSAD) is synthesized. With the incorporation of acetylenedicarboxylate (AD) units
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.