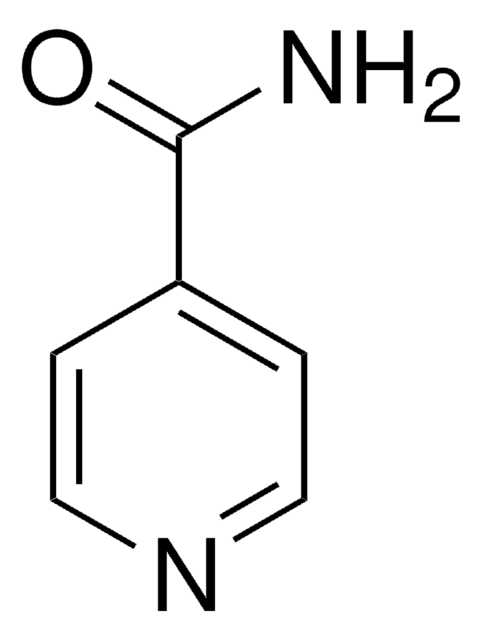
추천 제품
Quality Level
제품 라인
ReagentPlus®
분석
99%
mp
155-157 °C (lit.)
SMILES string
NC(=O)c1ccncc1
InChI
1S/C6H6N2O/c7-6(9)5-1-3-8-4-2-5/h1-4H,(H2,7,9)
InChI key
VFQXVTODMYMSMJ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
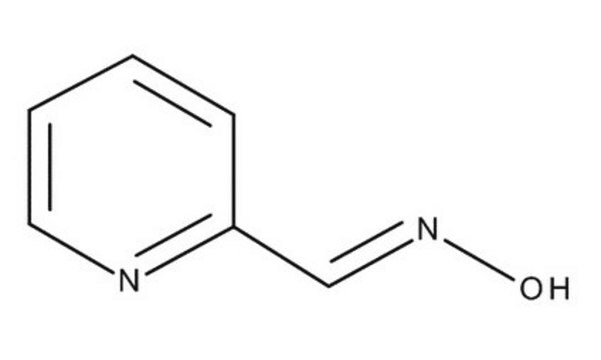
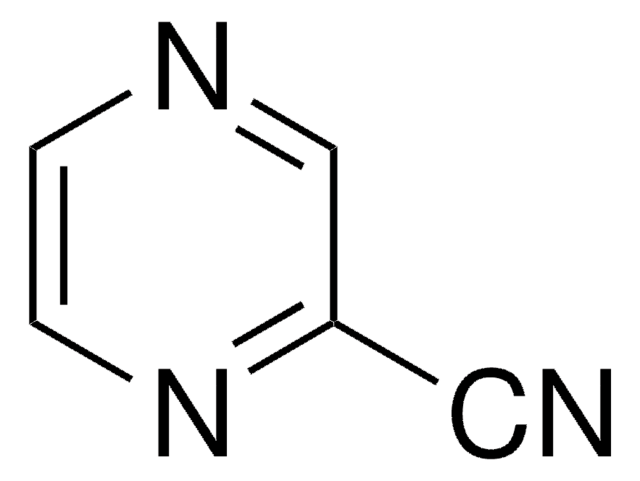
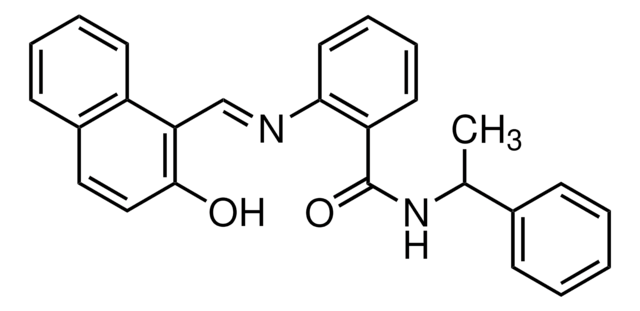
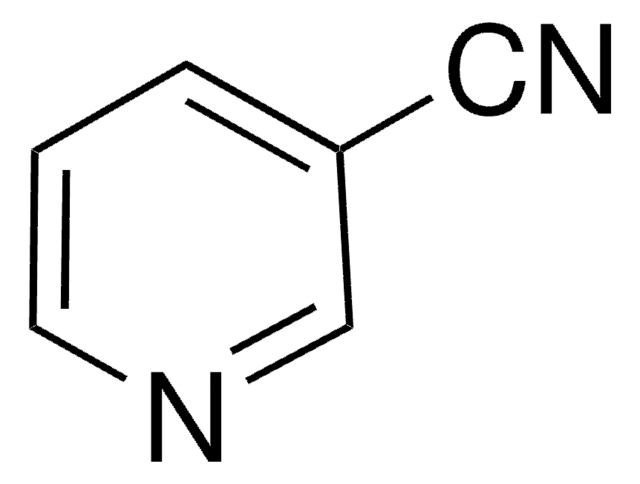
Isonicotinamide (pyridine-4-carboxamide) can be used as a heterocyclic building block to synthesize:
It can also be used as a co-former with active pharmaceutical ingredients (APIs) to prepare co-crystals.
- 4-oxo-1,3-thiazinan-3-yl isonicotinamide derivatives as potential anti-tubercular agents.
- Organotin(IV) complexes of isonicotinamide via synthesis of phosphoramidate ligands for various biological activity studies.
- Bis-pyridinium isonicotinamide derivatives of 2-(hydroxyimino)-N-(pyridin-3-yl)acetamide as potent reactivators sarin.
It can also be used as a co-former with active pharmaceutical ingredients (APIs) to prepare co-crystals.
법적 정보
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
QSAR, docking studies of 1, 3-thiazinan-3-yl isonicotinamide derivatives for antitubercular activity
Chitre TS, et al.
Computational Biology and Chemistry, 68, 211-218 (2017)
New organotin (IV) complexes of nicotinamide, isonicotinamide and some of their novel phosphoric triamide derivatives: Syntheses, spectroscopic study and crystal structures
Gholivand K, et al.
Journal of Organometallic Chemistry, 695(9), 1383-1391 (2010)
Gareth Arnott et al.
Organic letters, 10(14), 3089-3092 (2008-06-17)
Treatment of N-arylisonicotinamides with trifluoromethanesulfonic anhydride triggers intramolecular nucleophilic attack of the aryl ring on the 4-position of the pyridinium intermediate. The products are spirocyclic dihydropyridines which can be converted to valuable spirocyclic piperidines related to biologically active molecules such
Shaun R Stauffer et al.
Bioorganic & medicinal chemistry letters, 17(6), 1788-1792 (2007-01-30)
A series of low-molecular weight 2,6-diamino-isonicotinamide BACE-1 inhibitors containing an amine transition-state isostere were synthesized and shown to be highly potent in both enzymatic and cell-based assays. These inhibitors contain a trans-S,S-methyl cyclopropane P(3) which bind BACE-1 in a 10s-loop
Rodrigo A de Souza et al.
European journal of medicinal chemistry, 45(11), 4863-4868 (2010-08-21)
Complexes of the type trans-[PdX(2)(isn)(2)] {X = Cl (1), N(3) (2), SCN (3), NCO (4); isn = isonicotinamide} were synthesized and evaluated for in vitro antimycobacterial and antitumor activities. The coordination mode of the isonicotinamide and the pseudohalide ligands was
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.