추천 제품
Quality Level
분석
≥97%
색상
light yellow
mp
215 °C (dec.) (lit.)
저장 온도
−20°C
SMILES string
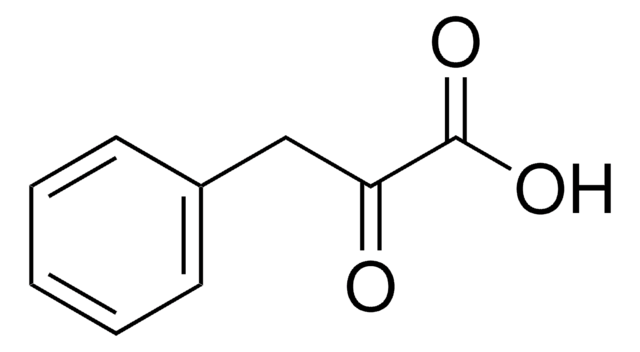
OC(=O)C(=O)Cc1c[nH]c2ccccc12
InChI
1S/C11H9NO3/c13-10(11(14)15)5-7-6-12-9-4-2-1-3-8(7)9/h1-4,6,12H,5H2,(H,14,15)
InChI key
RSTKLPZEZYGQPY-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Indole-3-pyruvic acid can be used:
- As a precursor for the synthesis of chromopyrrolic acid by a heme-containing enzyme.
- As a reactant in the Biginelli-like scaffold syntheses.
결합
α-Keto analogue of tryptophan
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
L Bacciottini et al.
Pharmacological research communications, 19(11), 803-817 (1987-11-01)
The effects of acute or repeated administration of indole-pyruvic acid (IPA), a keto-analogue of tryptophan (TRP), were studied in various brain areas of rats by measuring the changes of 5-hydroxytryptamine (5-HT) and of norepinephrine (NE) content and metabolism. The analgesic
Direct formation of chromopyrrolic acid from indole-3-pyruvic acid by StaD, a novel hemoprotein in indolocarbazole biosynthesis
Asamizu, S, et al.
Tetrahedron Letters, 47(4), 473-475 (2006)
Goutam Chowdhury et al.
Chemical research in toxicology, 22(12), 1905-1912 (2009-10-29)
Aerobic incubation of the tryptophan transamination/oxidation product indole-3-pyruvic acid (I3P) at pH 7.4 and 37 degrees C yielded products with activity as Ah receptor (AHR) agonists. The extracts were fractionated using HPLC and screened for AHR agonist activity. Two compounds
Nathan D Tivendale et al.
Plant physiology, 159(3), 1055-1063 (2012-05-11)
Seeds of several agriculturally important legumes are rich sources of the only halogenated plant hormone, 4-chloroindole-3-acetic acid. However, the biosynthesis of this auxin is poorly understood. Here, we show that in pea (Pisum sativum) seeds, 4-chloroindole-3-acetic acid is synthesized via
Cyclic ketones and substituted α-keto acids as alternative substrates for novel Biginelli-like scaffold syntheses
Abelman, Matthew M, et al.
Tetrahedron Letters, 44(24), 4559-4562 (2003)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.