I5508
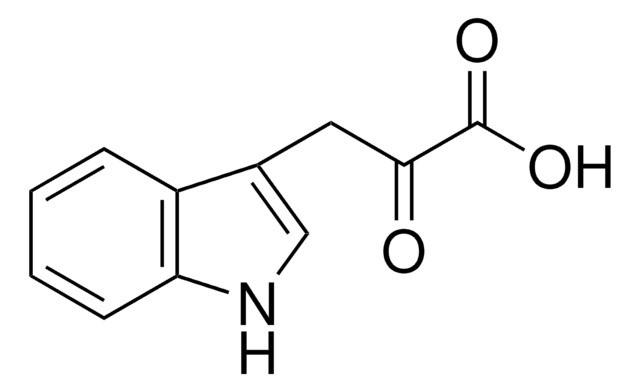
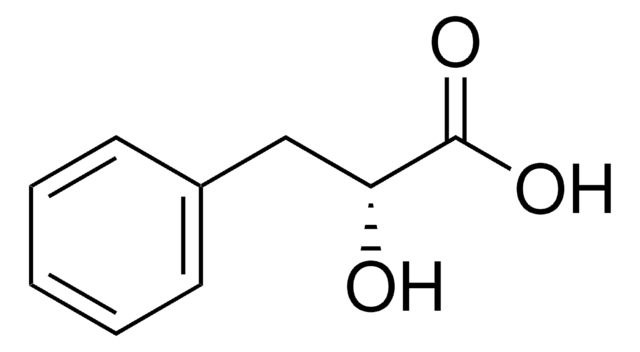
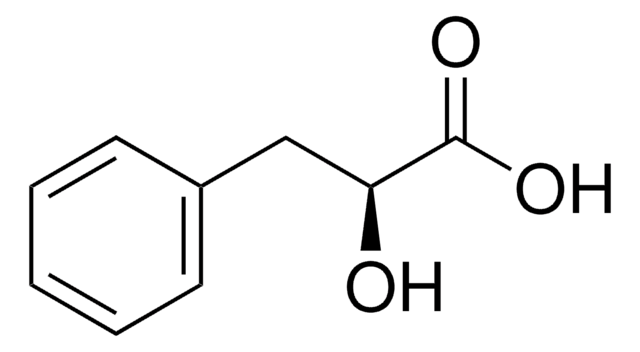
DL-Indole-3-lactic acid
99%
Synonym(s):
β-(3-Indolyl)lactic acid, 2-Hydroxy-3-(3-indolyl)propanoic acid, 3-(3-Indolyl)-2-hydroxypropanoic acid, 3-Indolyllactic acid, Indolelactic acid
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(1)
Select a Size
Change View
About This Item
Empirical Formula (Hill Notation):
C11H11NO3
CAS Number:
Molecular Weight:
205.21
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
99%
mp
145-146 °C (lit.)
SMILES string
OC(Cc1c[nH]c2ccccc12)C(O)=O
InChI
1S/C11H11NO3/c13-10(11(14)15)5-7-6-12-9-4-2-1-3-8(7)9/h1-4,6,10,12-13H,5H2,(H,14,15)
InChI key
XGILAAMKEQUXLS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
DL-Indole-3-lactic acid can serve as a building block in the synthesis of various molecules, like Indole-3-acetic acid (IAA)
Application
Reactant for preparation of:
- Antibacterial agents
- Dietary sweetener
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Soumen K Manna et al.
Journal of proteome research, 9(8), 4176-4188 (2010-06-15)
Alcohol-induced liver disease (ALD) is a leading cause of nonaccident-related deaths in the United States. Although liver damage caused by ALD is reversible when discovered at the earlier stages, current risk assessment tools are relatively nonspecific. Identification of an early
Francisco A Moreno et al.
European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology, 20(1), 18-24 (2009-11-10)
The purpose of this study was to examine the differential effects of acute tryptophan (TRP) depletion vs. sham condition on plasma, cerebrospinal fluid (CSF) biochemical parameters, and mood in the following three subject groups: (1) nine antidepressant-free individuals with remitted
Determination of urinary 5-hydroxyindole-3-acetic acid using solid-phase extraction and reversed-phase high-performance liquid chromatography with electrochemical detection.
P P Chou et al.
Journal of chromatography, 341(1), 167-171 (1985-05-31)
Magdalena Hilbert et al.
The New phytologist, 196(2), 520-534 (2012-08-29)
Beneficial effects elicited by the root endophyte Piriformospora indica are widely known, but the mechanism by which these are achieved is still unclear. It is proposed that phytohormones produced by the fungal symbiont play a crucial role in the interaction
H Körber et al.
The EMBO journal, 10(13), 3983-3991 (1991-12-01)
Oncogenes carried by the transferred DNA (T-DNA) of Agrobacterium Ti plasmids encode the synthesis of plant growth factors, auxin and cytokinin, and induce tumour development in plants. Other T-DNA genes regulate the tumorous growth in ways that are not yet
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service