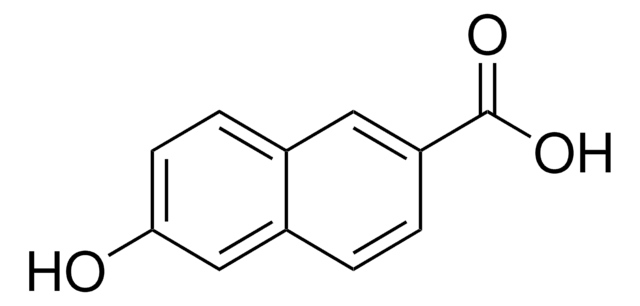
추천 제품
애플리케이션
1-Naphthoic acid can be used as a reactant to prepare:
- Perinaphthenones by dehydrative annulation with alkynes in the presence of rhodium catalyst.
- Isocoumarin derivatives by reacting with 2-butyne via aerobic oxidative cyclization using Rh catalyst.
- N-Methoxy-N-methyl-1-naphthalenecarboxamide (Weinreb amide) by reacting with N,O-dimethylhydroxylamine and phosphorus trichloride.
- 1,4-Dihydro-1-naphthalenecarboxylic acid by Birch reduction.
기타 정보
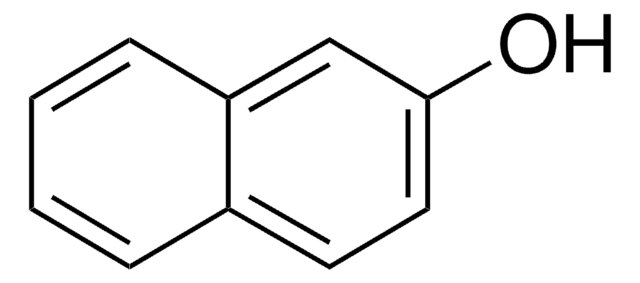
Remainder 2-naphthoic acid
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Hiromasa Uchiyama et al.
European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 43(1-2), 71-77 (2011-04-06)
Spray-dried particles (SDPs) with indomethacin (IND) and alpha-glycosyl transferase-treated stevia (Stevia-G) indicated extremely high dissolution rates and apparent solubility compared to particles of a ground mixture and a physical mixture of IND/Stevia-G. The apparent solubility of IND from SDPs was
Synthesis of perinaphthenones through rhodium-catalyzed dehydrative annulation of 1-naphthoic acids with alkynes
Fukuyama T, et al.
Organic & Biomolecular Chemistry, 16, 7583-7587 (2018)
S Chandra et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 74(3), 704-713 (2009-09-02)
The Fourier transform infrared gas phase spectrum of Naphthoic acid (NA) was recorded in the region 4000-400 cm(-1). The Fourier transform Raman spectrum and Fourier transform IR spectra of NA were recorded in solid phase. Quantum chemical calculations of energies
Rajesh Sunasee et al.
The Journal of organic chemistry, 73(20), 8016-8020 (2008-09-26)
A method is described for converting tert-butyl benzoates or tert-butyl 1-naphthoates into derivatives having an alkyl or substituted alkyl group in a 1,4-relationship to an alkyl, aryl, alkenyl, or alkynyl group. Key steps in the sequence are (i) addition of
Qunfei Zhao et al.
Chemistry & biology, 15(7), 693-705 (2008-07-19)
Azinomycin B is a complex natural product containing densely assembled functionalities with potent antitumor activity. Cloning and sequence analysis of the azi gene cluster revealed an iterative type I polyketide synthase (PKS) gene, five nonribosomal peptide synthetases (NRPSs) genes and
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.