P1101
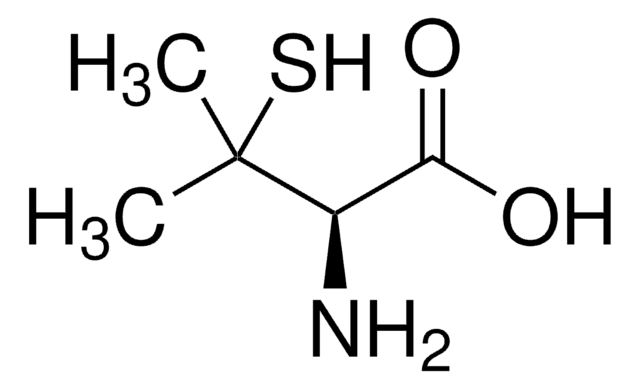
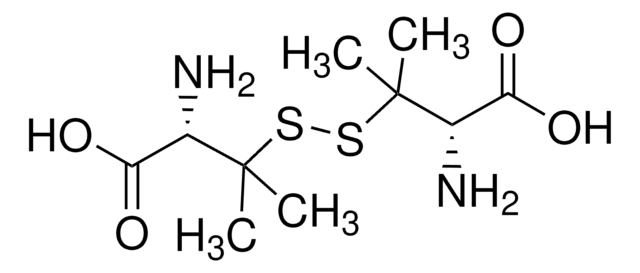
D-Penicillamine disulfide
97%, cell analysis
동의어(들):
3,3′-Dithiobis(2-amino-3-methylbutanoic acid), 3,3′-Dithiobis(2-amino-3-methylbutyric acid), 3,3′-Dithiobis-D-valine, S,S′-Bi(D-penicillamine), NSC 87505
About This Item
추천 제품
product name
D-Penicillamine disulfide, 97%
Quality Level
분석
97%
형태
powder or crystals
광학 활성
[α]25/D −75°, c = 1 in 1 M NaOH
반응 적합성
reaction type: solution phase peptide synthesis
색상
white
mp
204 °C (dec.) (lit.)
응용 분야
cell analysis
SMILES string
CC(C)(SSC(C)(C)[C@@H](N)C(O)=O)[C@@H](N)C(O)=O
InChI
1S/C10H20N2O4S2/c1-9(2,5(11)7(13)14)17-18-10(3,4)6(12)8(15)16/h5-6H,11-12H2,1-4H3,(H,13,14)(H,15,16)/t5-,6-/m0/s1
InChI key
POYPKGFSZHXASD-WDSKDSINSA-N
애플리케이션
- Molecules dermatomyositis activity
- Antimelanoma activity of apoptogenic carbonyl scavengers
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.