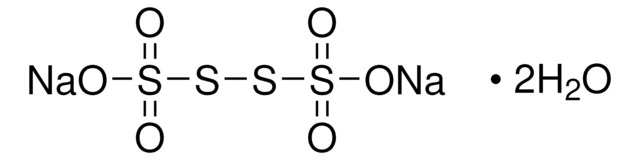모든 사진(3)
About This Item
Linear Formula:
KOSO2SSSO3K
CAS Number:
Molecular Weight:
302.45
EC Number:
MDL number:
UNSPSC 코드:
12352302
PubChem Substance ID:
NACRES:
NA.23
추천 제품
일반 설명
Potassium tetrathionate is a potassium salt of tetrathionic acid. Potassium tetrathionate is typically found as a white or off-white crystalline solid. It is soluble in water. We offer it for research purpose with a purity of ≥ 98 %. It is a sulfuric acid dimerformed by a disulfide linkage. It is widely used in the field of microbiology as aningredient for tetrathionate broth.
애플리케이션
Potassium tetrathionate, K2S4O6, is widely used in research and development (R&D), especially in the field of chemistry and biology. Some of the notable uses of it are : It acts as an oxidizing agent during chemical reactions. It readily reduces to thiosulfate which makes it a useful reducing agent in a wide range of oxidation-reduction systems. It can be used as a reagent in analytical methods in the determination of few compounds. For example: the iodometric determination of traces of iodine in solution. In Material Science it may be applied to the synthesis of sulfur containing material, or in the corrosion studies of metals by the sulfur compounds. In one of the studies, it has been used to test stress corrosion cracking of thermo-mechanically processed microstructures. It has been used as a precursor for electroless gold plating solution which is used in printed circuit boards (PCBs) to prevent Nickel corrosion. Catalysis: Due to its incorporation in redox processes, it could be employed for catalytical or co-catalytical purposes in some chemical reactions. Environmental Studies- It finds utility in environmental studies, for example in quantification of sulfur compounds in soil, water, and air samples. In biology, potassium tetrathionate may be used as a reagent in studies of sulfur metabolism in microorganisms. It has been used in studies concerning microbial sulfur oxidation pathways. It finds applications in textile industry as bleaching agent. Potassium tetrathionate (K2S4O6) can be used as an energy source for the batch culture of Acidithiobacillus caldus. K2S4O6 can be used as an additive (pit prevent agent) to prepare an electrolyte composition for electroplating of Cu-Sn alloy. It can also be used to study the adsorption competition of lead and sulfur on nickel in acidic aqueous media. K2S4O6 is preferred over other sulfur species as it is an acid-stable source of S and does not produce a large quantity of H2S.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
Zhanna Bugaytsova et al.
European journal of biochemistry, 271(2), 272-280 (2004-01-14)
The moderately thermophilic bacterium Acidithiobacillus caldus is found in bacterial populations in many bioleaching operations throughout the world. This bacterium oxidizes elemental sulfur and other reduced inorganic sulfur compounds as the sole source of energy. The purpose of this study
A new method for predicting susceptibility of austenitic stainless steels to intergranular stress corrosion cracking
Rahimi S, et al.
Materials & Design, 187, 108368-108368 (2020)
Electrochemical study of S and Pb adsorption on Ni in aqueous acidic media
Ebrahimy A F, et al.
Corrosion Science, 175, 108878- 108878 (2020)
Structural health monitoring of stainless-steel nuclear fuel storage canister using acoustic emission
Ai Li. et al.
Developments in the Built Environment, 17, 100294-100294 (2024)
Daniel B Grabarczyk et al.
PloS one, 12(3), e0173395-e0173395 (2017-03-04)
The Sox pathway found in many sulfur bacteria oxidizes thiosulfate to sulfate. Pathway intermediates are covalently bound to a cysteine residue in the carrier protein SoxYZ. We have used biochemical complementation by SoxYZ-conjugates to probe the identity of the intermediates
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.