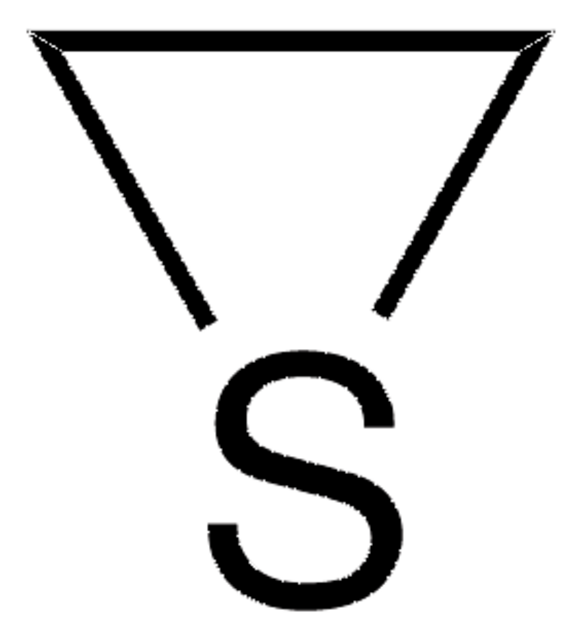
추천 제품
애플리케이션
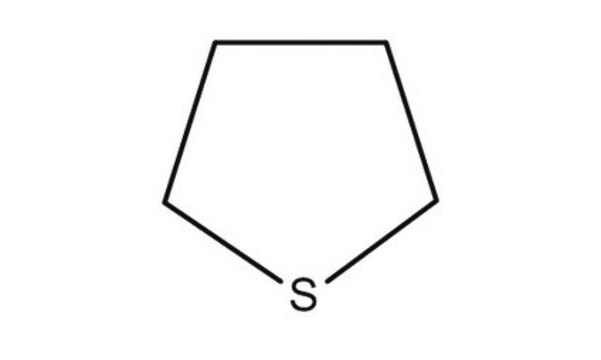
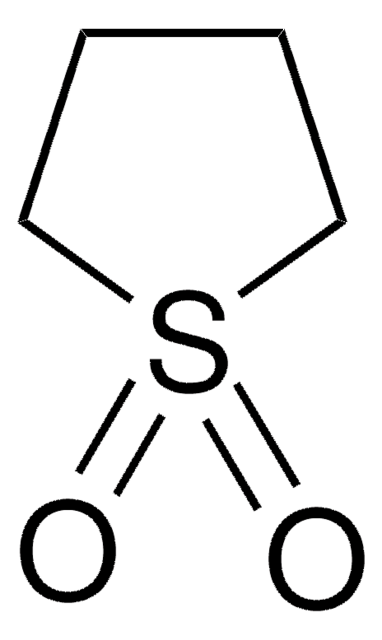
Tetrahydrothiophene can be used as a reagent for the synthesis of various epoxides and their derivatives. It can be used as a catalyst for the synthesis of benzo[n.1.0]bicycloalkanes.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point (°F)
55.4 °F - closed cup
Flash Point (°C)
13 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Catalytic asymmetric synthesis of epoxides from aldehydes using sulfur ylides with in situ generation of diazocompounds.
Aggarwal VK, et al.
Angewandte Chemie (International Edition in English), 40(8), 1430-1433 (2001)
Tetrahydrothiophene-catalyzed synthesis of benzo [n.1.0] bicycloalkanes.
Ye LW, et al.
The Journal of Organic Chemistry, 72(4), 1335-1340 (2007)
A new protocol for the in situ generation of aromatic, heteroaromatic, and unsaturated diazo compounds and its application in catalytic and asymmetric epoxidation of carbonyl compounds. Extensive studies to map out scope and limitations, and rationalization of diastereo-and enantioselectivities.
Aggarwal VK, et al.
Journal of the American Chemical Society, 125(36), 10926-10940 (2003)
Sanat Ghosh et al.
Physical chemistry chemical physics : PCCP, 22(31), 17482-17493 (2020-06-13)
This is a tale of a pair of a hydrogen bond donor and acceptor, namely the CH donor and sulphur acceptor, neither of which is a conventional hydrogen bond participant. Sulfur (S), being less electronegative (2.58) compared to its first
Irena Martin-Kleiner et al.
Cell biochemistry and function, 26(8), 916-919 (2008-10-23)
NEP/CALLA or CD10 is an endopeptidase (E.C. 3.4.24.11) that inactivates numerous neuropeptides, including dynorphin. Dynorphin is an endogenous opioid polypeptide that binds to kappa-opioid receptors with greatest affinity. R1.1 mouse thymoma cells highly express kappa-opioid receptors. In this study, on
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.