W342106
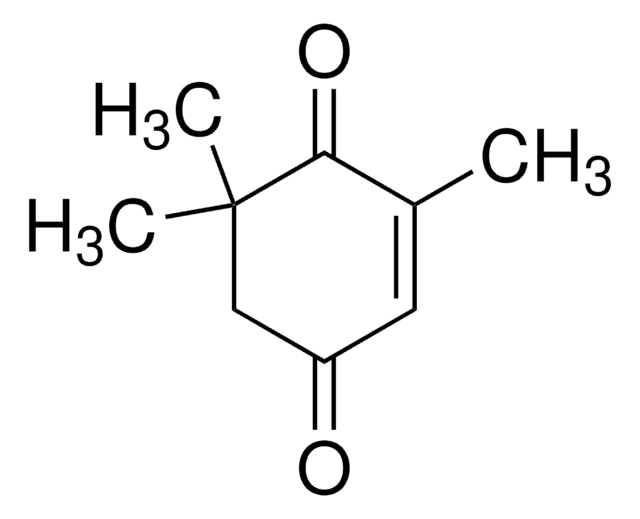
4-Oxoisophorone
≥98%, FG
동의어(들):
2,6,6-Trimethyl-2-cyclohexene-1,4-dione, 4-Oxoisophorone, Ketoisophorone
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C9H12O2
CAS Number:
Molecular Weight:
152.19
FEMA Number:
3421
Beilstein:
2207030
EC Number:
유럽평의회 번호:
11200
MDL number:
UNSPSC 코드:
12164502
PubChem Substance ID:
플래비스(Flavis) 번호:
7.109
NACRES:
NA.21
감각 수용성의:
musty; woody; sweet
Grade:
FG
Fragrance grade
Halal
Kosher
Fragrance grade
Halal
Kosher
생물학적 소스:
synthetic
Agency:
follows IFRA guidelines
meets purity specifications of JECFA
meets purity specifications of JECFA
식품 알레르기항원:
no known allergens
추천 제품
생물학적 소스
synthetic
Quality Level
Grade
FG
Fragrance grade
Halal
Kosher
Agency
follows IFRA guidelines
meets purity specifications of JECFA
규정 준수
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
분석
≥98%
refractive index
n20/D 1.491 (lit.)
bp
222 °C (lit.)
92-94 °C/11 mmHg (lit.)
mp
26-28 °C (lit.)
응용 분야
flavors and fragrances
문건
see Safety & Documentation for available documents
식품 알레르기항원
no known allergens
향수 알레르기항원
no known allergens
감각 수용성의
musty; woody; sweet
SMILES string
CC1=CC(=O)CC(C)(C)C1=O
InChI
1S/C9H12O2/c1-6-4-7(10)5-9(2,3)8(6)11/h4H,5H2,1-3H3
InChI key
AYJXHIDNNLJQDT-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
4-Oxoisophorone is a flavor compound found in tobacco and saffron.
애플리케이션
<ul>
<li><strong>Characterization of Evodia rutaecarpa (Juss) Benth honey: volatile profile, odor-active compounds and odor properties.</strong> This study investigates the unique aroma compounds in Evodia rutaecarpa honey, identifying 4-Oxoisophorone as a key component influencing its sensory characteristics, crucial for developing enhanced food products with distinct flavors (Li et al., 2024).</li>
<li><strong>Enantioselective Total Synthesis of (R,R)-Blumenol B and d(9)-(R,R)-Blumenol B.</strong> Describes the synthesis of biologically active compounds using 4-Oxoisophorone as a precursor, demonstrating its versatility in chemical synthesis and potential applications in developing pharmaceutical agents (Tan et al., 2022).</li>
<li><strong>The mystery of the butterfly bush Buddleja davidii: How are the butterflies attracted </strong> Explores the chemical attractants of Buddleja davidii, with 4-Oxoisophorone identified as a significant attractant, which could have implications for enhancing biodiversity and garden ecology (Lehner et al., 2022).</li>
</ul>
<li><strong>Characterization of Evodia rutaecarpa (Juss) Benth honey: volatile profile, odor-active compounds and odor properties.</strong> This study investigates the unique aroma compounds in Evodia rutaecarpa honey, identifying 4-Oxoisophorone as a key component influencing its sensory characteristics, crucial for developing enhanced food products with distinct flavors (Li et al., 2024).</li>
<li><strong>Enantioselective Total Synthesis of (R,R)-Blumenol B and d(9)-(R,R)-Blumenol B.</strong> Describes the synthesis of biologically active compounds using 4-Oxoisophorone as a precursor, demonstrating its versatility in chemical synthesis and potential applications in developing pharmaceutical agents (Tan et al., 2022).</li>
<li><strong>The mystery of the butterfly bush Buddleja davidii: How are the butterflies attracted </strong> Explores the chemical attractants of Buddleja davidii, with 4-Oxoisophorone identified as a significant attractant, which could have implications for enhancing biodiversity and garden ecology (Lehner et al., 2022).</li>
</ul>
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
개인 보호 장비
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Study on the flavor change of tobacco powder during enzymatic hydrolysis and Maillard reaction
Wen DM, et al.
Science and Technology of Food Industry, 12, 038-038 (2012)
Worldwide market screening of saffron volatile composition.
Maggi L, et al.
Journal of the Science of Food and Agriculture, 89(11), 1950-1954 (2009)
Mohamed-Elamir F Hegazy et al.
Zeitschrift fur Naturforschung. C, Journal of biosciences, 63(5-6), 403-408 (2008-08-02)
Stereospecific olefin (C=C) and carbonyl (C=O) reduction of the readily available prochiral compound ketoisophorone (2,2,6-trimethyl-2-cyclohexene-1,4-dione) (1) by Marchantia polymorpha and Nicotiana tabacum cell suspension cultures produce the chiral products (6R)-levodione (2), (4R,5S)-4-hydroxy-3,3,5-trimethylcyclohexanone (3), and (4R,6R)-actinol (4) as well as the
Stefano Raimondi et al.
Journal of biotechnology, 156(4), 279-285 (2011-09-22)
Old yellow enzymes (OYEs, EC 1.6.99.1) are flavin-dependent oxidoreductases that catalyze the stereoselective trans-hydrogenation of the double bond, representing a promising alternative to metal-based catalysis. Bioconversion of ketoisophorone (KIP) by 28 non-conventional yeasts belonging to 16 different species was investigated.
Matthias Lechtenberg et al.
Planta medica, 74(7), 764-772 (2008-05-23)
Extracts from saffron, the dried stigmata from Crocus sativus L., are being used more and more in preclinical and clinical trials for the treatment of cancer and depression. Because of the known quality problems of saffron, HPLC methods on RP(18)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.