모든 사진(1)
About This Item
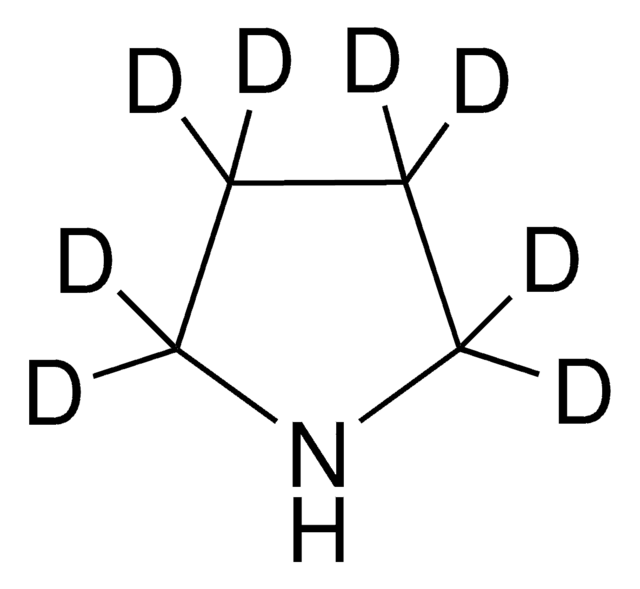
실험식(Hill 표기법):
C4H9N
CAS Number:
Molecular Weight:
71.12
FEMA Number:
3523
Beilstein:
102395
EC Number:
MDL number:
UNSPSC 코드:
12164502
PubChem Substance ID:
플래비스(Flavis) 번호:
14.064
NACRES:
NA.21
감각 수용성의:
fishy
Grade:
FG
Halal
Halal
생물학적 소스:
synthetic
Agency:
meets purity specifications of JECFA
식품 알레르기항원:
no known allergens
추천 제품
생물학적 소스
synthetic
Quality Level
Grade
FG
Halal
Agency
meets purity specifications of JECFA
규정 준수
EU Regulation 1334/2008 & 178/2002
vapor density
2.45 (vs air)
vapor pressure
128 mmHg ( 39 °C)
49 mmHg ( 20 °C)
autoignition temp.
653 °F
expl. lim.
10.6 %
refractive index
n20/D 1.443 (lit.)
density
0.852 g/mL at 25 °C (lit.)
응용 분야
flavors and fragrances
문건
see Safety & Documentation for available documents
식품 알레르기항원
no known allergens
감각 수용성의
fishy
SMILES string
C1CCNC1
InChI
1S/C4H9N/c1-2-4-5-3-1/h5H,1-4H2
InChI key
RWRDLPDLKQPQOW-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1A
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point (°F)
37.4 °F - closed cup
Flash Point (°C)
3 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Joseph T Paletta et al.
Organic letters, 14(20), 5322-5325 (2012-10-12)
A series of sterically shielded pyrrolidine nitroxides were synthesized, and their reduction by ascorbate (vitamin C) indicate that nitroxide 3, a tetraethyl derivative of 3-carboxy-PROXYL, is reduced at the slowest rate among known nitroxides, i.e., at a 60-fold slower rate
Adele Faulkner et al.
Chemical communications (Cambridge, England), 49(15), 1521-1523 (2013-01-17)
We report efficient Pd-catalyzed cyclizations of oxime esters with 1,1-disubstituted alkenes as the basis of a general entry to α,α-disubstituted pyrrolidine derivatives. We also demonstrate that catalytic asymmetric variants of this chemistry are feasible by employing a suitable chiral ligand.
Pieter Van der Veken et al.
Journal of medicinal chemistry, 55(22), 9856-9867 (2012-11-06)
We have investigated the effect of regiospecifically introducing substituents in the P2 part of the typical dipeptide derived basic structure of PREP inhibitors. This hitherto unexplored modification type can be used to improve target affinity, selectivity, and physicochemical parameters in
Boyu Zhang et al.
Angewandte Chemie (International ed. in English), 51(52), 13159-13162 (2012-11-20)
Drop it! A highly enantioselective catalytic cascade reaction of α-ketoacids and aldehydes is achieved using the title catalyst and water as the solvent. Fluorescence imaging shows that the catalyst is mainly distributed on the surface of emulsion droplets. Optically active
Hiroaki Chiba et al.
Angewandte Chemie (International ed. in English), 51(36), 9169-9172 (2012-08-15)
In control: The novel and enantioselective total synthesis of (-)-quinocarcin includes the highly stereoselective preparation of the 2,5-cis-pyrrolidine by intramolecular amination, a selective substrate-controlled 6-endo-dig intramolecular alkyne hydroamination with a cationic Au(I) catalyst, and Lewis-acid-mediated ring-opening/halogenation sequence.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.