8.18203
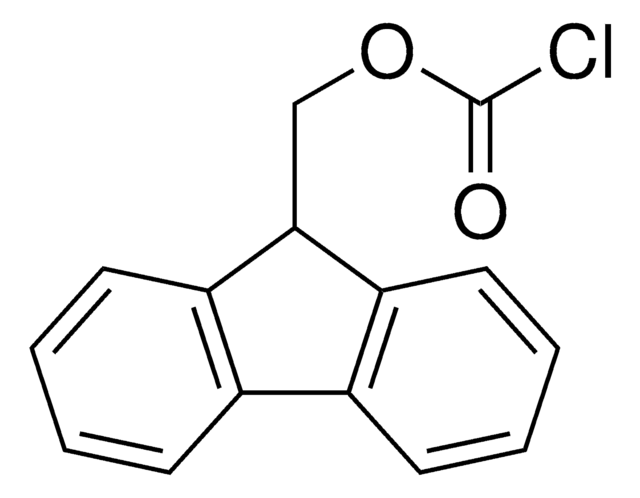
(9-Fluorenylmethyl) chloroformate
for synthesis
동의어(들):
(9-Fluorenylmethyl) chloroformate, Chloroformic acid 9-fluorenylmethyl ester, FMOCCl
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C15H11ClO2
CAS Number:
Molecular Weight:
258.70
MDL number:
UNSPSC 코드:
12352108
EC 인덱스 번호:
249-313-6
NACRES:
NA.22
응용 분야:
peptide synthesis
추천 제품
Quality Level
양식
powder
mp
60-63 °C
응용 분야
peptide synthesis
저장 온도
15-25°C
SMILES string
ClC(=O)OCC1c2c(cccc2)c3c1cccc3
InChI
1S/C15H11ClO2/c16-15(17)18-9-14-12-7-3-1-5-10(12)11-6-2-4-8-13(11)14/h1-8,14H,9H2
InChI key
IRXSLJNXXZKURP-UHFFFAOYSA-N
일반 설명
(9-Fluorenylmethyl) chloroformate, also known as Fmoc chloride, is a highly versatile reagent with varied applications in organic synthesis. It is most frequently used to introduce the base-labile Fmoc-protecting group to amine functionalities, particularly during the production of Fmoc-protected amino acids. Fmoc-chloride has also been used to generate mixed carboxylic and carbonic anhydrides to facilitate amide and ester bond formation. It is soluble in common organic solvents such as dichloromethane, tetrahydrofuran, and dimethylformamide.
애플리케이션
Recent applications of (9-Fluorenylmethyl) chloroformate include:
- (9-Fluorenylmethyl) chloroformate can be used as a coupling reagent.
- In the synthesis of amino acid esters. Fmoc chloride activates the carboxylic acid group of the amino acid, allowing it to react with an alcohol to form the ester.
- In the preparation of lysogangliosides.
- In the solid-phase peptide synthesis of DOPA-containing peptides.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
보충제 위험성
Storage Class Code
8A - Combustible, corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Synthesis of Reactive Amino Acid Esters by Using 9-Fluorenylmethyl Chloroformate
Yamamoto H and Wu A
Synfacts, 16, 1247-1247 (2020)
9-Fluorenylmethoxycarbonyl amino-protecting group
Carpino LA and Han GY
The Journal of Organic Chemistry, 37, 3404-3404 (1972)
Synthesis of peptides containing DOPA (3, 4-dihydroxyphenylalanine)
Sever MJ and Wilker JJ
Tetrahedron, 6139-6146 (2001)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.