추천 제품
Grade
ACS reagent
Quality Level
양식
powder
구성
Dye content, 95%
색상
pink-purple
visual transition interval
6.0-7.6, yellow to blue(passes test)
mp
200-202 °C ((392 - 396 °F) - lit.)
200-202 °C (lit.)
λmax
420 nm
ε (흡광계수)
≥17000 at 419-425 nm in methanol
≥8000 at 326-332 nm in methanol
≥9000 at 279-285 nm in methanol
저장 온도
room temp
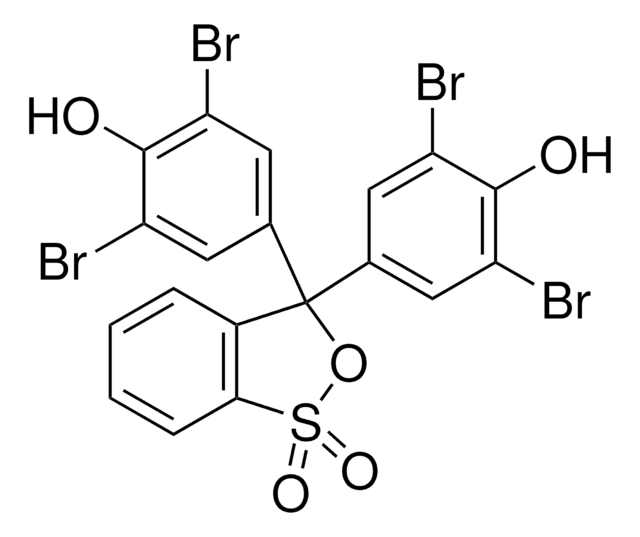
SMILES string
CC(C)c1cc(c(C)c(Br)c1O)C2(OS(=O)(=O)c3ccccc23)c4cc(C(C)C)c(O)c(Br)c4C
InChI
1S/C27H28Br2O5S/c1-13(2)17-11-20(15(5)23(28)25(17)30)27(19-9-7-8-10-22(19)35(32,33)34-27)21-12-18(14(3)4)26(31)24(29)16(21)6/h7-14,30-31H,1-6H3
InChI key
NUHCTOLBWMJMLX-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
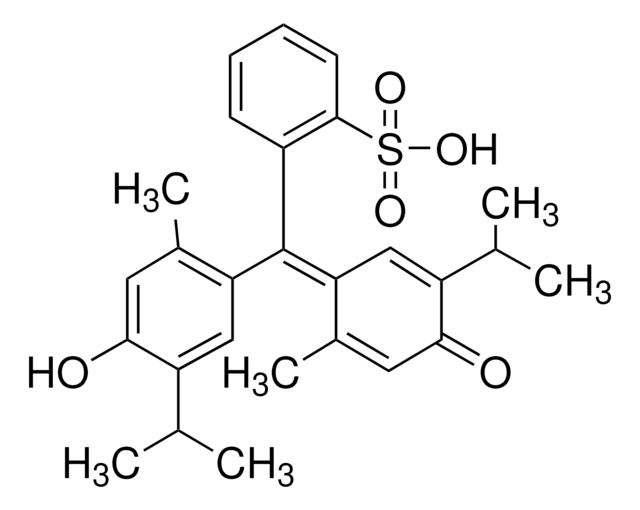
일반 설명
Bromothymol Blue, also known as, dibromothymolsulfonphthalein is a sulfonated hydroxyquinone in acidic aqueous solutions. In alkaline solutions, this dye is present as a moderately sized dianion. It is also known as bromthymol blue and dibromothymolsulfonphthalein. It is a pH-sensitive dye that changes color from yellow to blue.
애플리케이션
Bromothymol blue dye serves as a pH indicator because of the precise color transition at neutrality. Other applications include:
- as a vital stain to trace the movement of fluids from the lymph and to define cell walls or nuclei under the microscope
- for the demonstration of fungal hyphae within plant roots
- in the assessment of the soluble acidity within single rice grains
- use in an agar gel medium for enumeration of Bacillus cereus
- as a component of several bacterial growth and detection substrates such as cystine lactose electrolyte deficient (CLED/BROLACIN); polymyxin pyruvate egg yolk mannitol bromothymol blue (PEMBA) and MacConkey agar.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
Andrea Pastore et al.
Talanta, 219, 121251-121251 (2020-09-06)
A pH colorimetric sensor array (CSA) with fast response time (<1 min) using only one acid-base indicator, Bromothymol Blue (BB), was prepared and characterized by modulating the amount, C, of the surfactant Hexadecyltrimethylammonium p-toluenesulfonate between 0 and 0.3725 gCTApTs/gprecursor with a
Reza Amin et al.
Micromachines, 11(6) (2020-07-01)
To transform from reactive to proactive healthcare, there is an increasing need for low-cost and portable assays to continuously perform health measurements. The paper-based analytical devices could be a potential fit for this need. To miniaturize the multiplex paper-based microfluidic
Hisataka Maruyama et al.
Lab on a chip, 8(2), 346-351 (2008-01-31)
This paper demonstrates local pH measurement in a microchip using a pH-sensing gel-microbead. To achieve this, the gel-microbead made of a hydrophilic photo-crosslinkable resin was functionalized with the pH indicator bromothymol blue (BTB). The primary constituent of this photo-crosslinkable resin
Mariana A Sanchez et al.
Analytica chimica acta, 694(1-2), 95-99 (2011-05-14)
Liquid-liquid microextraction without phase segmentation was implemented in a multicommuted flow system for determination of the anti-hypertensive diltiazem. The procedure was based on ion pair formation between the drug and the dye bromothymol blue at pH 3.5. The detection was
Kristina Jurcic et al.
Journal of chromatography. A, 1134(1-2), 317-325 (2006-10-07)
An effective sample preconcentration technique for proteins and peptides was recently developed using capillary electrophoresis (CE) with discontinuous buffers [C.A. Nesbitt, J.T.-M. Lo, K.K.-C. Yeung, J. Chromatogr. A 1073 (2005) 175]. Two buffers of different pH created a junction to
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.