추천 제품
Grade
analytical standard
Quality Level
제품 라인
VETRANAL®
유통기한
limited shelf life, expiry date on the label
기술
HPLC: suitable
gas chromatography (GC): suitable
응용 분야
forensics and toxicology
pharmaceutical (small molecule)
veterinary
형식
neat
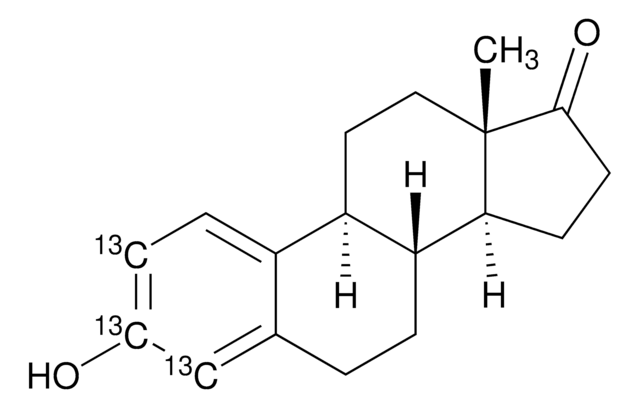
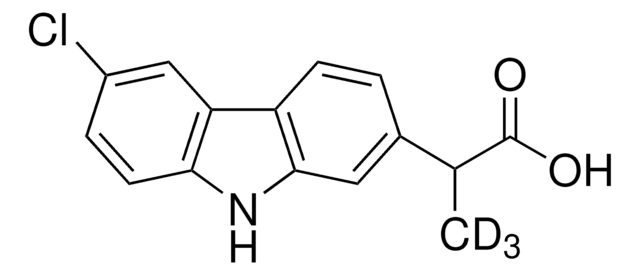
SMILES string
[2H]C([2H])([2H])C(C(O)=O)c1cccc(c1)C(=O)c2ccccc2
InChI
1S/C16H14O3/c1-11(16(18)19)13-8-5-9-14(10-13)15(17)12-6-3-2-4-7-12/h2-11H,1H3,(H,18,19)/i1D3
InChI key
DKYWVDODHFEZIM-FIBGUPNXSA-N
애플리케이션
Ketoprofen-d3 has been used as a surrogate analytical standard for the determination of ketoprofen in sewage sludge samples by QuEChERS (quick, easy, cheap, effective, rugged and safe) extraction with on-line solid-phase extract purification and pre-concentration, followed by liquid chromatography-tandem mass spectrometry (LC-MS/MS).
It may be used as an analytical standard for the determination of ketoprofen in compost from sewage sludge by ultrasound-assisted extraction (UAE) and salt-assisted liquid–liquid extraction (SALLE), followed by ultrahigh performance liquid chromatography (UHPLC) coupled to MS/MS.
It may be used as an analytical standard for the determination of ketoprofen in compost from sewage sludge by ultrasound-assisted extraction (UAE) and salt-assisted liquid–liquid extraction (SALLE), followed by ultrahigh performance liquid chromatography (UHPLC) coupled to MS/MS.
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
법적 정보
VETRANAL is a registered trademark of Merck KGaA, Darmstadt, Germany
관련 제품
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Sara Castiglioni et al.
Journal of hazardous materials, 384, 121441-121441 (2019-10-22)
In this work we evaluated the contamination of the water cycle in Como Bay by measuring 38 selected pharmaceuticals in two main wastewater treatment plant in Switzerland and in Italy, two influents (River Breggia and Cosia), lake water (epilimnion and
Jaber Assaf et al.
Journal of pharmaceutical and biomedical analysis, 145, 414-422 (2017-07-22)
Investigations on the photochemical stability of pharmaceutical substances are mandatory in drug development and licensing as photo-induced degradation of an active pharmaceutical ingredient (API) may not only lead to decreased API concentrations but also to toxic or reactive products. Thus
C Roos et al.
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 132, 222-230 (2018-09-30)
The number of highly lipophilic active pharmaceutical ingredients (APIs) in pharmaceutical development has been constantly increasing over recent decades. These APIs often have inherent issues with solubility and dissolution, limiting their oral bioavailability. Traditionally, a reduction in particle size to
C Roos et al.
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 142, 387-395 (2019-07-16)
Oral administration of drug products is the preferred administration route. In recent decades there has been an increase in drug candidates with low solubility and/or low permeability. To increase the possibility of oral administration for the poorly permeating drugs, the
Melissa M Melough et al.
Journal of agricultural and food chemistry, 65(24), 5049-5055 (2017-06-06)
Furocoumarins are a class of photoactive compounds found in several plant species and may be responsible for the observed association between consumption of citrus products and the risk of skin cancer. Furocoumarin contents of several foods have been reported previously
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.